

A Model of Integrative Care for Low-back Pain This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Altern Complement Med. 2012 (Apr); 18 (4): 354–362 ~ FULL TEXT
OPEN ACCESS David M. Eisenberg, MD, Julie E. Buring, ScD, Andrea L. Hrbek, Roger B. Davis, ScD,
Maureen T. Connelly, MD, Daniel C. Cherkin, PhD, Donald B. Levy, MD,
Mark Cunningham, Bonnie O'Connor, PhD, and Diana E. Post, MD
Division of General Medicine and Primary Care,
Beth Israel Deaconess Medical Center,
Harvard Medical School,
Boston, MA 02115, USA.
David_Eisenberg@hms.harvard.edu
FROM: Weeks ~ JMPT 2016 (Feb) Hurwitz ~ JMPT 2016 (May)OBJECTIVES: While previous studies focused on the effectiveness of individual complementary and alternative medical (CAM) therapies, the value of providing patients access to an integrated program involving multiple CAM and conventional therapies remains unknown. The objective of this study is to explore the feasibility and effects of a model of multidisciplinary integrative care for subacute low-back pain (LBP) in an academic teaching hospital.
DESIGN: This was a pilot randomized trial comparing an individualized program of integrative care (IC) plus usual care to usual care (UC) alone for adults with LBP.
SUBJECTS: Twenty (20) individuals with LPB of 3-12 weeks' duration were recruited from an occupational health clinic and community health center.
INTERVENTIONS: Participants were randomized to 12 weeks of individualized IC plus usual care versus UC alone. IC was provided by a trained multidisciplinary team offering CAM therapies and conventional medical care.
OUTCOME MEASURES: The outcome measures were symptoms (pain, bothersomeness), functional status (Roland-Morris score), SF-12, worry, and difficulty performing three self-selected activities.
RESULTS: Over 12 weeks, participants in the IC group had a median of 12.0 visits (range 5-25). IC participants experienced significantly greater improvements at 12 weeks than those receiving UC alone in symptom bothersomeness (p=0.02) and pain (p=0.005), and showed greater improvement in functional status (p=0.08). Rates of improvement were greater for patients in IC than UC in functional status (p=0.02), bothersomeness (p=0.002), and pain scores (p=0.001). Secondary outcomes of self-selected most challenging activity, worry, and the SF-12 also showed improvement in the IC group at 12 weeks. These differences persisted at 26 weeks, but were no longer statistically significant.
CONCLUSIONS: It was feasible for a multidisciplinary, outpatient IC team to deliver coordinated, individualized intervention to patients with subacute LBP. Results showed a promising trend for benefit of treating patients with persistent LBP with this IC model, and warrant evaluation in a full-scale study.
From the FULL TEXT Article:
Introduction
Four nationally representative surveys conducted between 1990 and 2007 demonstrated that a third or more of U.S. adults routinely use complementary and alternative medical (CAM) therapies to treat their principal medical conditions. [1–4] Total visits to CAM practitioners exceeded 300 million annually. [1, 2, 4] Total expenditures for CAM therapies were estimated at $14 billion in 1990, [1] $27 billion in 1997, [2] and $34 billion in 2007. [4] Out-of-pocket expenditures for CAM therapies in 2007 accounted for 11% of all out-of-pocket health care expenditures. [4] Despite the popularity of and substantial expenditures on CAM therapies, their effect on clinical outcomes and health care costs remains controversial. [5–8]
Low-back pain (LBP) is the most common medical condition for which adults use CAM therapies. [1, 2] BP is common, costly, and clinically challenging. An estimated 50% of adults experience significant LBP annually, and 70%—80% of adults are afflicted by LBP at some time in their lives. [9–11] In 1998, total health care expenditures incurred by individuals with LBP were estimated at $91 billion (1% of gross domestic product) with incremental expenditures attributable to back pain estimated at $26 billion. [12] These estimates excluded short- and long-term disability costs. Additional worker productivity losses due to pain-related conditions (with LBP as one of the three most costly) have been estimated at $61 billion per year. [13] In addition, wide variation in the medical and surgical management of LBP reflects widespread professional uncertainty about optimal care for this common condition. [14, 15]
Among adults with LBP surveyed in 1997–1998, 59% saw both a medical doctor and used one or more CAM modalities. [2] In addition, nearly one third of all visits to CAM providers in 1997–1998 were made for the treatment of back or neck pain. [2, 16, 17] The evidence-based Clinical Guidelines jointly published by the American College of Physicians and the American Pain Society identified eight treatments for chronic LBP with “moderate” evidence of effectiveness (none had strong evidence), including four CAM therapies: acupuncture, massage, spinal manipulation, and yoga. [18]
However, these studies evaluated individual CAM modalities as opposed to multiple CAM (and conventional) modalities, as is commonplace in real-life settings. [19] Moreover, while other investigators have studied the effectiveness of various multidisciplinary approaches to the treatment of LBP, these have emphasized conventional medical and psychologic treatments with little or no use of CAM therapies as key components of their “integrative care teams.” [20–24]
To address this gap in knowledge, a pilot randomized trial was designed to explore the effectiveness of an individualized program of multidisciplinary, integrative care — which included CAM practitioners — plus usual care compared to usual care alone for adults with LBP. It is hypothesized that coordinated access to both conventional and CAM practices delivered by a team trained to provide individualized, multidisciplinary care will provide superior outcomes for patients with subacute LBP.
Materials and Methods
Study population
Recruitment occurred between July 2004 and October 2005 at Harvard Vanguard Medical Associates, a large multi-specialty group practice in Boston, and at the Occupational Health Department of Brigham and Women's Hospital (BWH). Study procedures were approved by the human subjects committees of Harvard Vanguard Medical Associates, Partners Healthcare, and Harvard Medical School.
Patients 18–70 years old, presenting to these sites for an evaluation of work-related or non-work-related LBP, were screened for eligibility by a clinician. Exclusions included LBP for less than 21 or more than 84 days; pain above the lower back; LBP too mild (<3 when asked: “During the past week, on a scale of 0–10 where 0=no pain and 10=worst pain ever, how bad is your back pain?”); history of back surgery in the last 3 years; history of vertebral fracture or dislocation; progressive or severe neurological symptoms; known spondylolisthesis, scoliosis, or ankylosing spondylitis; pacemaker or implanted defibrillator; underlying systemic or visceral disease causing back pain; known osteoporosis; taking systemic corticosteroids; pregnancy (suspected or known); history of cancer (other than nonmelanoma skin cancer) within past 5 years; unexplained weight loss or recent unexplained fever; bleeding disorder or taking anticoagulant medication; severe or disabling co-existing problem; major organ transplantation; immunosuppressive medication; intravenous drug use; unable to speak or understand English; or unavailable for appointments within study guidelines.
Study outcomes
Follow-up interviews were conducted by telephone at 2, 5, 12, and 26 weeks. Interviewers were not blinded, but performed all interviews according to a predetermined script. Primary outcomes were defined as changes from baseline to 12 weeks in functional status, symptom relief, and pain. Functional status was measured using the modified Roland-Morris Disability Questionnaire, [25, 26] which includes 23 yes–no questions about daily activities such as difficulty getting dressed and climbing stairs (23 = maximal dysfunction). Symptom relief was measured as bothersomeness of the worst symptom (LBP, sciatica, or numbness) over the past 24 hours on a 0–10 scale (0 = not bothersome, 10 = extremely bothersome). [27, 28] LBP was measured by asking subjects about their worst pain over the prior 24 hours using a 0–10 scale, with 0 = no pain and 10 = worst pain ever.
Secondary outcomes included worry about one's back problem and overall mental and physical health. Worry was measured using a scale from 0 (no worry) to 10 (extremely worried). The Medical Outcomes Study Short-Form (SF-12, acute version) was used to measure overall mental and physical health, on subscales ranging from 0 (worst) to 100 (best). [29, 30] In addition, participants were asked at baseline to identify three important activities in their daily life that LBP or sciatica had made difficult, and to rate the difficulty of each on a 0–10 scale.
Intervention
Eligible patients with subacute LBP were randomized in a 2:1 ratio to integrative care plus usual care (IC refers to this adjunctive intervention group throughout the article) versus usual care (UC) alone.
Participants randomized to usual medical care (UC) continued to receive treatment at their primary care facilities, typically including nonsteroidal anti-inflammatory drugs, muscle relaxants, as-needed referrals to physical therapy, limited bed rest, education, and activity alterations.>
Integrative Care (IC) was provided by a trained multidisciplinary team with licensed practitioners who could provide the following services if needed:acupuncture,
chiropractic,
internal medicine consultation and referral as appropriate,
massage therapy,
occupational therapy,
physical therapy,
mind–body techniques,
neurology consultation,
nutritional counseling,
orthopedics consultation, and
psychiatry and rheumatology consultation and referrals as appropriate.See Figure 1 for overview of the study design.
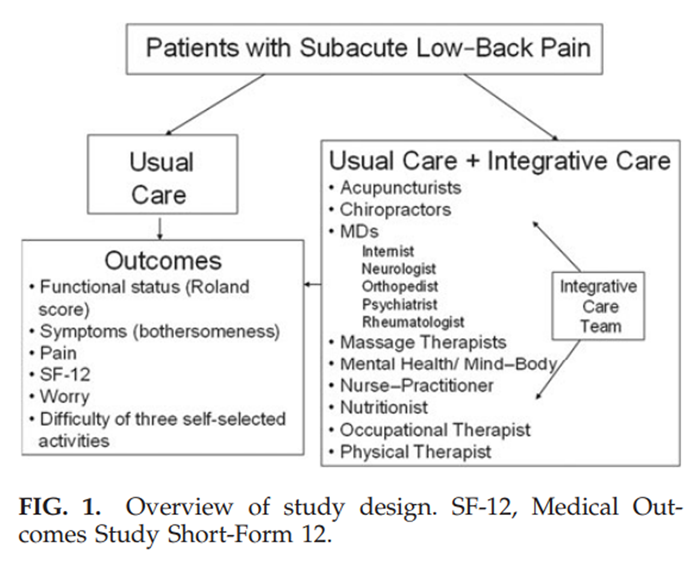
Figure 1 The team was trained one full day per week for 14 consecutive weeks to refer nonhierarchically to fellow team members to maximally enhance a patient's functionality with the fewest encounters. Training was co-led by a professional facilitator, a medical anthropologist and the team's medical director, an internist, and consisted of
(1) didactic presentations by each member;
(2) experiential education including hands on diagnosis and treatment by each member on other team members;
(3) the diagnosis and treatment of volunteer subjects with chronic LBP, referred by team member physicians, in full view of all team members; and
(4) the development of a shared treatment protocol for implementation of the pilot randomized controlled trial.
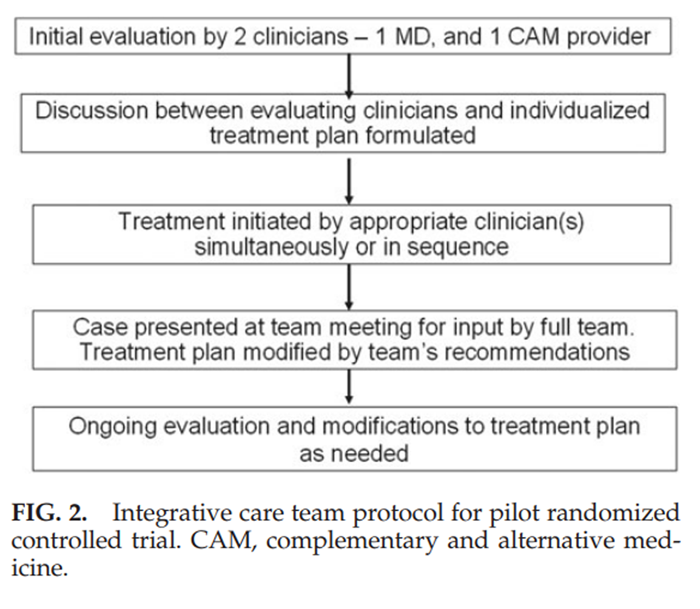
Figure 2 Participants were evaluated at the initial visit by a medical doctor and CAM clinician (Fig. 2). The two evaluators then met to develop an individualized treatment plan. Treatments were provided up to 2 times per week (with up to 2 treatment modalities per session) for up to 12 weeks as needed. Cases were discussed at weekly team meetings. Team members were encouraged to refer across disciplines (listed in Fig. 1) to maximally reduce symptoms and enhance patient function (i.e., capacity to perform routine tasks), and to prospectively engage each patient in participatory therapies to the extent accepted by each patient. The shared goal was to help each patient achieve maximal improvement with a minimum number of visits over a 12-week period. Type and frequency of treatments provided were recorded. Scope-of-practice guidelines were established for staff chiropractors, acupuncturists, and massage therapists, consistent with their professional scope of practice in Massachusetts. However, the integrative care team did not prescribe herbs, vitamins, or supplements as part of the study, nor did they perform cervical manipulation, consistent with hospital guidelines at the time the study was implemented.
Statistical analysis
For each outcome, the IC versus UC groups were compared using Wilcoxon rank-sum tests evaluating the change in score from baseline. To examine the rate of improvement over the 12-week treatment period, linear regression models were created. These included the baseline value of the outcome, a treatment-group indicator, study week, and a treatment-group–study-week interaction term as covariates. Generalized estimating equations with an autoregressive correlation structure were used to account for within-participant correlation. Likelihood ratio tests were employed to evaluate whether the rates of improvement were different between treatment groups. All analyses were performed on an intention-to-treat basis. Planned analyses were conducted using last value carried forward to replace missing data. To ensure that results were not artifacts of this approach, all analyses were repeated using only observed data. None of the results was substantively changed. Data reported here are based on the planned, intention-to-treat analyses. In this pilot study, no adjustments were made for multiple tests.
Results
Baseline
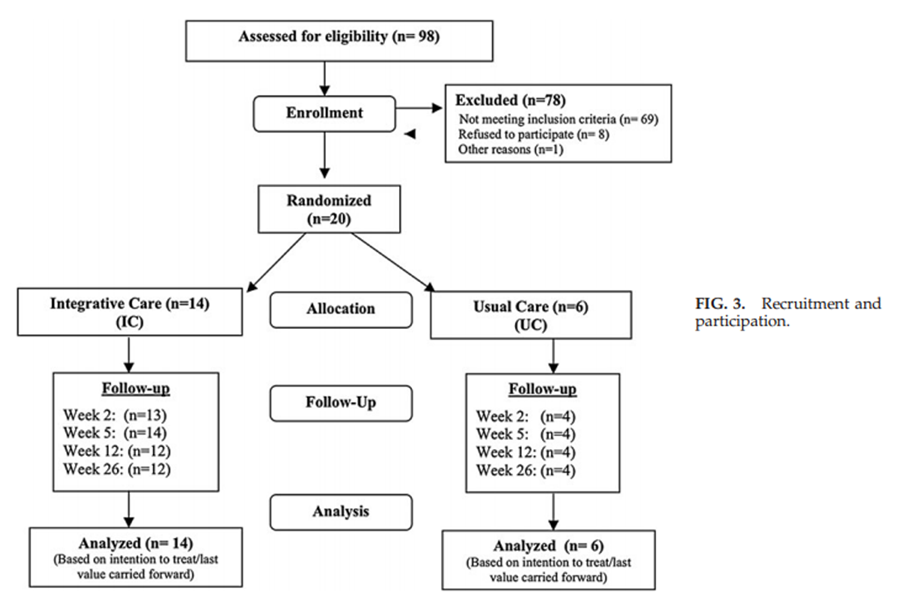
Figure 3 Ninety-eight (98) patients were screened for the study (Fig. 3). Of these, 8 were eligible but declined to participate, and 70 were ineligible, primarily due to chronicity of pain (n=30), pain too mild (6), age not in range (5), not an employee or member of the health plan participating (6), or for a history of one of several additional exclusionary conditions (23), including osteoporosis (4); scoliosis, spondyolisthesis, or ankylosing spondylitis (3); not able to be enrolled in time window (3); not LBP (2); infrequent pain (2); prior spine surgery (2); spinal fracture (1); fibromyalgia (1); skin cancer (1); pacemaker (1); steroid use (1); intravenous drug use (1); and pregnancy (1). The 20 participants who remained eligible and willing were randomized in a 2:1 ratio, 14 to IC and 6 to UC.
Main outcomes

Table 1 All baseline measures were obtained prior to randomization (Table 1).
The two groups were well balanced at baseline except for the following:(1) percentage involved with Worker's Compensation claim (higher number in IC group);
(2) number of days spent in bed (higher in UC group); and
(3) satisfaction with baseline “usual care” (higher in UC group).

Table 2
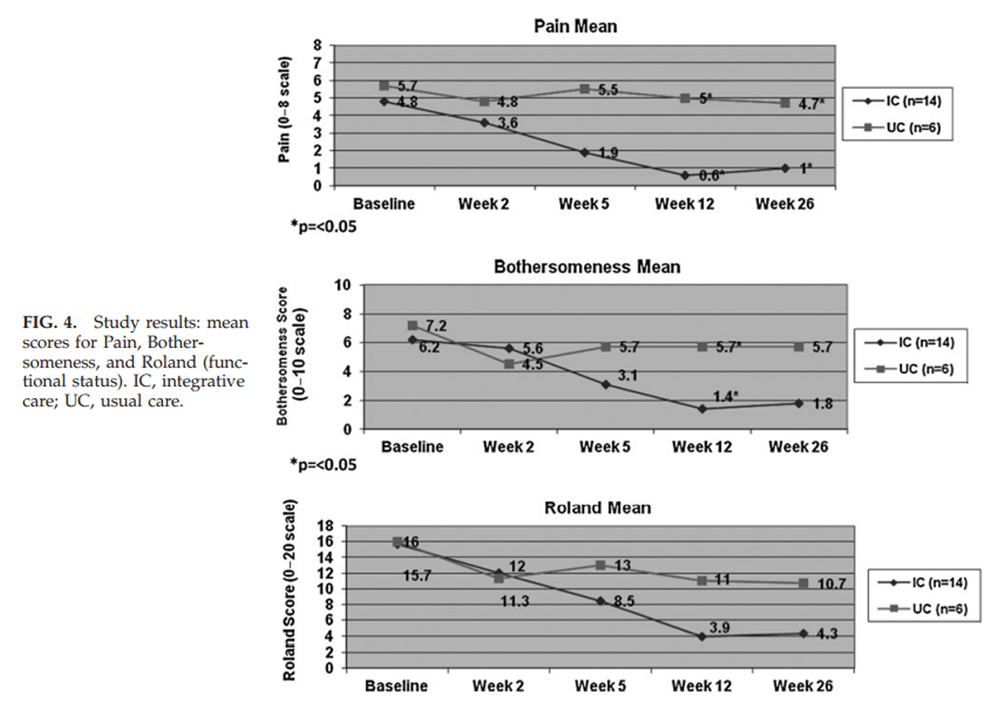
Figure 4

Table 3 Over 12 weeks, participants in the IC group had a median of 12.0 visits (range of 5–25). One minor adverse event was reported (pain at an acupuncture site) out of over 170 CAM treatments provided to the IC group. As shown in Table 2 and Figure 4, at 12 weeks, there were statistically significant benefits in the IC group compared to UC with regard to mean pain scores (0.6 versus 5, p=0.005), and bothersomeness (1.4 versus 5.7, p=0.02). Mean functional status (Roland score) was substantially better in the IC group (3.9 versus 11), but did not reach statistical significance (p=0.08).
These differences between groups narrowed slightly at 26 weeks and were no longer statistically significant, except for pain. The secondary outcomes of self-selected most challenging activity, worry, and the SF-12 physical scale also showed benefit in the IC group at 12 weeks.
A secondary analysis using longitudinal regression showed that IC participants experienced significantly greater improvement in the three primary outcomes over 12 weeks (Table 3). Functional status (Roland score) improved by an average of 0.89 points per week (95% confidence interval: 0.61, 1.17) for IC compared to 0.32 (–0.004, 0.64) for UC (p=0.02); bothersomeness improved by 0.39 (0.29, 0.48) points per week for IC compared to 0.05 (–0.08, 0.19) for UC (p=0.002); and pain scores improved by 0.30 (0.24, 0.37) points per week for IC compared to 0.04 (–0.10, 0.19) for UC (p=0.001). The benefits remained but were no longer statistically significant at 26 weeks.
Discussion
LBP is a common, costly, and suboptimally managed condition. It is also a prime candidate for studies that explore the effectiveness and cost-effectiveness, or lack thereof, of “integrative care models” that combine access to both conventional and complementary care options. The results of this prospective pilot randomized trial address the hypothesis that coordinated access to a trained multidisciplinary, outpatient team consisting of medical doctors, allied health care personnel, and licensed CAM providers may result in enhanced clinical outcomes for adults with persistent LBP when compared to usual care alone.
The results suggest that(1) it is feasible to assemble and train a clinical team of conventional and licensed CAM providers within an academic teaching hospital;
(2) treatments delivered by CAM professionals within this model as applied to patients with LBP are safe; and
(3) access to an expanded multidisciplinary (i.e., integrative care) model may benefit patients with persistent LBP.However, confirmation of the findings of this pilot study in a fully powered trial needs to be established in order to consider possible implications for clinicians, economists, and self-insured corporations.
This pilot study has raised a number of issues that would need to be addressed in a full-scale trial designed to definitively evaluate these questions:(1) sufficient numbers of eligible patients with back pain would be required to ensure the comparability of the baseline characteristics between the treatment groups, as well as to provide adequate power to detect clinically meaningful treatment effects;
(2) there was a high loss to follow-up in the UC group, and approaches to maximize follow-up rates such as offers of financial incentives for completion of all outcome measures, which should ideally be applied to both UC and IC groups, need to be considered;
(3) blinding on the part of interviewers will be essential in any subsequent trial; and
(4) in this pragmatic study, there was no a priori algorithm delineating specific referral patterns.No 2 patients received identical patterns (i.e., “fingerprints”) or “doses” of “integrative care.” Indeed, this may not be dissimilar from the need for individualized, nonidentical care for individuals with a variety of complex, chronic medical conditions such as cancer, diabetes, or depression. A full-scale trial would need to balance the need for a reproducible intervention model that does not overly constrain clinicians caring for LBP patients, and the need to explore treatment patterns for subgroups of LBP patients to determine whether optimal treatment algorithms can be developed and prospectively tested.
A major limitation in this study relates to the likely differences between study arms regarding patient contact and communication with clinical providers. It is possible that the observed differences between groups were the result of enhanced patient contact, interaction, education, and encouragement by the several members of the integrative care team participating in each patient's treatment, and that this enhanced contact, as opposed to increased use of CAM therapies, was the “active ingredient” leading to the observed differences. Indeed, the results of studies by Karjalainen et al.20,21 clearly indicated that added to usual care, a mini-intervention by a physician specializing in back pain and a physiotherapist involving a clinical examination, information, support, and simple advice reduced daily symptoms and absenteeism, and led to better treatment satisfaction and adaptation to pain, compared with UC for patients with subacute LBP.
In another study intended to evaluate “an integrative care program” to treat subjects with subacute LBP, Lambeek et al., in studies reported in 2007, 2009, and 2010 [22-24] compared subjects (n=40) half of whom were randomized to receive usual care and half to receive a model of “integrated care” that combined a “patient-directed and workplace-directed” intervention provided -by a multidisciplinary team, including an occupational physician. The conclusions of the Lambeek et al. studies, like those of the Karjalainen et al. studies, included the observation that subjects treated by a multidisciplinary team experienced improved clinical outcomes as compared with “usual care.”
Importantly, however, both the Karjalainen and Lambeek studies excluded licensed CAM professionals (e.g., chiropractors, massage therapists, and acupuncturists) from their respective multidisciplinary teams. Ultimately, the question of which combination of licensed health care professionals, including both CAM and conventional providers, should ideally be involved in optimally cost-effective, multidisciplinary approaches to treat persistent lower back pain will remain unanswered until a fully powered trial with a comparable attention control such as integrated conventional care only is included. Lambeek et al. estimated that a sample of approximately 150 subjects would be needed for a properly powered study to address this question. [22]
Finally, a future trial needs to track not only clinical and functional outcomes, but also relevant financial outcomes including costs of patient visits, diagnostic tests, surgical procedures, medications, absenteeism, work status, worker productivity, short- and long- term disability payments, and employee replacement costs.
Evaluating these questions is important for two reasons. The first relates to the enormous societal costs of LBP and the need for novel therapeutic strategies to contain or reduce health care expenditures. Luo et al. reported that on average, individuals with LBP incur health care expenditures about 60% higher than individuals without LBP, with incremental expenditures attributable to the management of LBP in the United States accounting for 2.5% of all health care expenditures in 1998. [12] In a more recent analysis of the Medical Expenditure Panel Survey (n = 22,258), [31] investigators reported that total estimated expenditures among respondents with spine problems increased 65% from 1997 to 2005. However, age- and sex-adjusted measures of physical function, work limitations, social limitations, and mental health among LBP sufferers were all considerably worse in 2005 than in 1997. [31] Even a modest but statistically and clinically significant difference in symptoms, functional status, productivity, and utilization of services among LBP patients would have a significant impact on overall health care costs in the United States and could translate into a savings of billions of dollars annually.
Second, while the popularity of CAM therapies by U.S. adults is no longer debated, the role of these therapies in future models of coordinated, evidence-based, preference driven, fiscally responsible health care remains unclear. The Institute of Medicine, in its report entitled “Complementary and Alternative Medicine in the United States,” includes the following recommendation regarding the need for additional studies to evaluate “integrative models” of care: “Studies show that patients frequently do not limit themselves to a single modality of care—they do not see complementary and alternative medicine (CAM) and conventional medicine as being mutually exclusive— and this pattern will probably continue and may even expand as evidence of therapies effectiveness accumulate. Therefore, it is important to understand how CAM and conventional medical treatments (and providers) interact with each other and to study models of how the two kinds of treatments can be provided in coordinated ways. In that spirit, there is an urgent need for health systems research that focuses on identifying the elements of these integrative medical models, their outcomes and whether they are cost effective when compared to conventional practice.” [32]
Conclusions
Historically, federal agencies including the National Institutes of Health, the Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid, the Department of Defense, and the Veterans Administration have not sponsored research aimed at evaluating the cost effectiveness — or lack thereof — of emerging models of multidisciplinary, “integrative care” in the treatment of common medical conditions. This study argues that such comparative effectiveness research in this area is feasible, promising, and warranted, at least with regard to adults with persistent LBP.
Acknowledgments
This work was supported in part by grants from the National Center for Complementary and Alternative Medicine (AT00905, AT005065) and the Bernard Osher Foundation.Disclosure Statement
No competing financial interests exist.
References:
Eisenberg DM. Kessler RC. Foster C, et al.
Unconventional Medicine in the United States: Prevalence, Costs, and Patterns of Use
New England Journal of Medicine 1993 (Jan 28); 328 (4): 246–252Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC.
Trends in Alternative Medicine Use in the United States, 1990 to 1997:
Results of a Follow-up National Survey
JAMA 1998 (Nov 11); 280 (18): 1569–1575Barnes PM , Powell-Griner E , McFann K , Nahin RL:
Complementary and Alternative Medicine Use Among Adults:
United States, 2002
Advance Data 2004 (May 27); 343: 1–19Nahin RL. Barnes PM. Stussman BJ. Bloom B.
Costs of Complementary and Alternative Medicine (CAM)
and Frequency of Visits to CAM Practitioners:
United States, 2007
National Health Statistics Reports 2009 (Jul 30); (18): 1–14Maxion-Bergemeann S. Wolf M. Bornhoft G, et al.
Complementary and alternative medicine costs: A systemic literature review.
Forsch Komplementarmed. 2006;13(suppl 2):42–45White AR. Ernst E.
Economic analysis of complementary medicine: A systemic review.
Complement Ther Med. 2000;8:111–118Herman PM. Craig BM. Caspi O.
Is Complementary and Alternative Medicine (CAM) Cost-effective?
A Systematic Review
BMC Complementary and Alt Med 2005 (Jun 2); 5: 11Hulme C. Long AF.
Square pegs and round holes? A review of economic evaluation in complementary
and alternative medicine [see comment]
J Altern Complement Med. 2005;11:179–188Hart LG. Deyo RA. Cherkin DC.
Physician office visits for low back pain: Frequency, clinical evaluation,
and treatment patterns from a U.S. national survey.
Spine. 1995;20:11–19Deyo RA. Mirza SK. Martin BI.
Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002.
Spine. 2006;31:2724–2727Carey TS. Evans AT. Hadler NM, et al.
Acute severe low back pain: A population-based study of prevalence and care-seeking.
Spine. 1996;21:339–344Luo X, Pietrobon R, Sun SX, Liu GG, Hey L.
Estimates and Patterns of Direct Health Care Expenditures Among Individuals
With Back Pain in the United States
Spine (Phila Pa 1976) 2004 (Jan 1); 29 (1): 79–86Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R.
Lost Productive Time and Cost Due to Common Pain Conditions in the US Workforce
JAMA 2003 (Nov 12); 290 (18): 2443–2454Weinstein JNLJ. Tosteson TD. Skinner JS, et al.
Surgical vs nonoperative treatment for lumbar disk herniation:
The Spine Patient Outcomes Research Trial (SPORT) observational cohort.
JAMA. 2006;296:2451–2459Deyo RA.
Treatments for back pain: Can we get past trivial effects?
Ann Intern Med. 2004;141:957–958Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS:
Patterns and Perceptions of Care for Treatment of Back
and Neck Pain: Results of a National Survey
Spine (Phila Pa 1976) 2003 (Feb 1); 28 (3): 292–297Wolsko PM. Eisenberg DM. Davis RB, et al.
Insurance coverage, medical conditions, and visits to alternative medicine providers:
Results of a national survey.
Arch Intern Med. 2002;162:281–287Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492–504Guzman J. Esmail R. Karjalainen K, et al.
Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain.
Cochrane Database Syst Rev. 2002;1:CD000963Karjalainen K. Malmivaara A. Pohjolainen T, et al.
Mini-Intervention for subacute low back pain: A randomized controlled trial.
Spine. 2003;28:533–541Karjalainen K. Malmivaara A. Mutanen P, et al.
Mini-intervention for subacute low back pain: A two year follow-up
and modifiers of effectiveness.
Spine. 2004;29:1089–1076Lambeek LC. Anema JR. van Royen BJ, et al.
Multidisciplinary outpatient care program for patients with chronic low back pain:
A design of a randomized controlled trial and cost-effectiveness study.
BMC Public Health. 2007;7:254Lambeek LC. van Mechelen W. Buijs PC, et al.
An integrated care program to prevent work disability due to chronic low back pain:
A process evaluation within a randomized controlled trial.
BMC Musculoskel Disord. 2009;10:147Lambeek LC. Bosmans JE. van Royen B, et al.
Effect of integrated care for sick listed patients with chronic low back pain:
Economic evaluation alongside a randomized controlled trial.
BMJ. 2010;341:c6414Roland M. Morris R.
A study of the natural history of back pain: Part I.
Development of a reliable and sensitive measure of disability in low-back pain.
Spine. 1983;8:141–144Roland M. Morris R.
A study of the natural history of low-back pain: Part II.
Development of guidelines for trials of treatment in primary care.
Spine. 1983;8:145–150Patrick DL. Deyo RA. Atlas SJ, et al.
Assessing health-related quality of life in patients with sciatica.
Spine. 1995;20:1899–1908. discussion 1909Dunn KM. Croft PR.
Classification of Low Back Pain in Primary Care: Using "Bothersomeness"
to Identify the Most Severe Cases
Spine (Phila Pa 1976). 2005 (Aug 15); 30 (16): 1887–1892Ware J. Kosinksi M. Keller SD.
A 12-item short-form health survey: Construction of scales and preliminary tests
of reliability and validity.
Med Care. 1996;34:220–233Ware JE. Kosinksi M. Keller SD.
How to Score the SF-12 Physical and Mental Health Summary Scales. 2nd.
Worcester, MA: The Healthy Center,
New England Medical Center; 1995.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al.
Expenditures and Health Status Among Adults With Back and Neck Problems
JAMA 2008 (Feb 13); 299 (6): 656–664Institute of Medicine.
Complementary and Alternative Medicine in the United States PDF
Washington, DC: The National Academies Press; 2005.
Return to LOW BACK PAIN
Return to COST-EFFECTIVENESS
Return to INTEGRATED HEALTH CARE
Since 1-22-2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |