

Association Between Chiropractic Spinal Manipulation
and Gabapentin Prescription in Adults With Radicular
Low Back Pain: Retrospective Cohort Study Using US DataThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2023 (Jul 21); 13 (7): e073258 ~ FULL TEXT
OPEN ACCESS Robert J Trager, Zachary A Cupler, Roshini Srinivasan, Regina M Casselberry, Jaime A Perez, Jeffery A Dusek
Connor Whole Health,
University Hospitals Cleveland Medical Center,
Cleveland, Ohio, USA
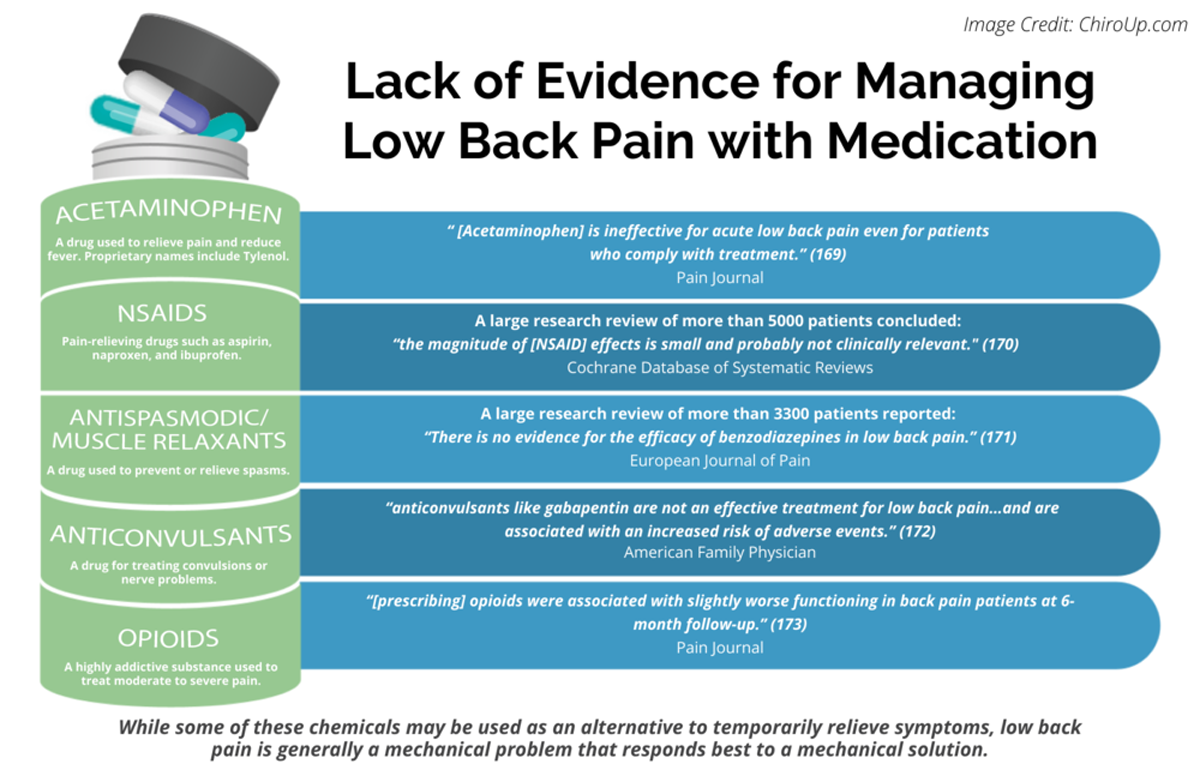
FROM: Pain 2019 (Dec)
FROM: Cochrane Database 2020 (Apr)
FROM: European Journal of Pain 2017 (Feb)
FROM: American Family Physician 2019 (Mar 15)
FROM: Pain 2013 (Jul)Objectives: Radicular low back pain (rLBP) is often treated off-label with gabapentin or by chiropractors using chiropractic spinal manipulative therapy (CSMT). To date, no studies have examined the association between these interventions. We hypothesised that adults under 50 years of age receiving CSMT for newly diagnosed rLBP would have reduced odds of receiving a gabapentin prescription over 1 year-follow-up.
Design: Retrospective cohort study.
Setting: US network including linked medical records, medical claims and pharmacy claims of >122 million patients attending large healthcare organisations (TriNetX), queried 15 June 2023, yielding data from 2017 to 2023.
Participants: Adults aged 18–49 were included at their first occurrence of rLBP diagnosis. Exclusions were severe pathology, other spinal conditions, on-label gabapentin indications and gabapentin contraindications. Propensity score matching controlled for variables associated with gabapentin use and receipt of prescription medication over the preceding year.
Interventions: Patients were divided into CSMT or usual medical care cohorts based on the care received on the index date of rLBP diagnosis.
Primary and secondary outcome measures: OR for gabapentin prescription.
Results: After propensity matching, there were 1635 patients per cohort (mean age 36.3±8.6 years, 60% women). Gabapentin prescription over 1–year follow-up was significantly lower in the CSMT cohort compared with the usual medical care cohort, with an OR (95% CI) of 0.53 (0.40 to 0.71; p<0.0001). Sensitivity analyses revealed early divergence in cumulative incidence of prescription; and no significant between-cohort difference in a negative control outcome (gastrointestinal medication) suggesting adequate control for pharmacological care preference.
Conclusions: Our findings suggest that US adults receiving CSMT for newly diagnosed rLBP have significantly reduced odds of receiving a gabapentin prescription over 1–year follow-up compared with those receiving usual medical care. Results may not be generalisable and should be replicated in other healthcare settings and corroborated by a prospective study to reduce confounding.
Keywords: complementary medicine; pain management; rehabilitation medicine
Strengths and limitations of this study
Study methods were crafted by an interdisciplinary team with the aim of minimising bias.
This study incorporated a new-user design, including patients at the first occurrence of a diagnosis of radicular low back pain, to make cohorts more homogeneous and comparable.
While we controlled for several variables via propensity matching to make cohorts more similar with respect to the likelihood of receiving a gabapentin prescription, variables such as income and pain severity were unavailable or poorly represented in the data set.
Although this study included several thousand patients, it may only be generalisable to large integrated academic healthcare settings in the USA.
Given that this study is observational and may have residual confounding, it should be repeated using a prospective study design.
From the FULL TEXT Article:
Background
The USA has the leading age-standardised prevalence of low back pain (LBP) in the world. [1] Together, low back and neck pain account for the leading cause of medical expenditures in the USA. [2] LBP can be divided into subtypes according to pathophysiology. Radicular low back pain (rLBP), which involves a nerve root lesion, is considered a type of neuropathic pain, and involves radiating symptoms into the ipsilateral lower extremity. [3, 4] Conversely, non-rLBP resulting from myofascial, discogenic, sacroiliac or zygapophyseal joint pain is considered nociceptive and does not necessarily radiate to the lower limb. [3, 4] Consequently, the subtype of LBP pathophysiology influences its pharmacological treatment approach. [5]
Gabapentin is an anticonvulsant, anti-epileptic medication, used as first-line therapy for several types of neuropathic pain including diabetic neuropathy and herpetic neuralgia. [6, 7] Gabapentin may alleviate neuropathic pain by binding to a subunit of voltage-gated calcium channels, subsequently inhibiting ectopic nerve discharges. [6, 7] Considering this mechanism of action, gabapentin has also been used off-label to treat neuropathic symptoms of LBP, namely rLBP. [5, 7]
While gabapentin has had supporting evidence and US Food and Drug Administration (FDA) approval for use in neuropathic pain conditions since 1993, [8, 9] systematic reviews in 2018 and 2022 demonstrated clear evidence of lack of its effectiveness for rLBP. [10, 11] Additionally, there is growing evidence of its risks including abuse, misuse, dependence and withdrawal. [9] Potentially deleterious adverse effects of gabapentin include somnolence, dizziness, ataxia and fatigue, as well as new-onset asthenic symptoms, particularly in patients with muscular problems. [12]
Accordingly, several clinical practice guidelines do not recommend gabapentin for the treatment of LBP or rLBP, [13] including those of the American Family Physician (2017). [14] Evidence supporting the use of gabapentin for LBP is considered inconclusive by guidelines from the North American Spine Society (2020), [15] Global Spine Care Initiative (2020) [16] and Veterans Affairs/Department of Defense (2019 and 2022). [17, 18] Furthermore, gabapentin prescription for LBP has been described as a marker of low-value care [19] and medical overuse. [20]
Despite the paucity of evidence, and in contrast to clinical guideline recommendations, gabapentin continues to be commonly prescribed for LBP. A survey of 545 US adults (mean age 52 years (range 20–92)) in 2018 revealed that 20% of patients who visited a medical doctor for LBP had been recommended gabapentin in the preceding 12 months. [21] A cross-sectional study examining over 230,000 outpatient visits in the USA between 2011 and 2015 found that 99% of gabapentin prescriptions were for off-label indications; the most common were degenerative spinal disorders and other back problems, together accounting for 27% of prescriptions. [22] In addition, there were increasing rates of episodes of prescription of gabapentin (relative increase of 440%) and concomitant opioid and gabapentin prescription (relative increase of 344%) in the USA between 2006 and 2018. [23]
Chiropractors are portal-of-entry providers in the USA who frequently treat spinal disorders. [24–26] When treating rLBP, these providers often use chiropractic spinal manipulative therapy (CSMT), [25] a hands-on treatment directed to the joints of the spine. [27] CSMT is supported by systematic reviews [28, 29] and recommended by clinical practice guidelines for the treatment of LBP [14, 15, 17] and rLBP. [30, 31]
Although chiropractors cannot prescribe medications such as gabapentin within their scope of practice, [24] previous studies have found that the initial type of provider seen for LBP influences the subsequent likelihood of receiving a prescription for certain medications. [32–34] These studies have found that patients initiating care for LBP with a chiropractor compared with other providers have reduced odds of receiving an opioid or benzodiazepine prescription. [33, 35, 36] However, to our knowledge, no research has explored the association between receipt of chiropractic care versus usual medical care for LBP and the likelihood of subsequent gabapentin prescription.
Considering that gabapentin is commonly prescribed off-label for rLBP, against spine and pain care guideline recommendations, the present study examined if undergoing CSMT influenced the subsequent likelihood of receiving a gabapentin prescription after rLBP diagnosis.
Objectives
This study examined the relationship between CSMT versus usual medical care and subsequent gabapentin prescription among patients newly diagnosed with rLBP identified from a large US database. We hypothesised that adults receiving CSMT on the index date of rLBP diagnosis would have reduced odds of receiving a gabapentin prescription compared with those receiving usual, non-chiropractic medical care over 1–year follow-up.
Materials and methods
Study design
This study incorporated a retrospective observational cohort design using aggregated and linked medical records, medical claims and pharmacy claims data, and implemented new-user, active comparator features to improve cohort comparability and reduce bias. [37, 38] An a priori protocol for the present study was registered in the Open Science Framework in January 2023 (https://osf.io/rt6f3). [39] Our manuscript reporting adheres to the Strengthening the Reporting of Observational Studies in Epidemiology statement. [40]
Following peer review at BMJ Open, we made three changes to our methods in June 2023, in which we(1) added a cumulative incidence graph to illustrate the timing of gabapentin prescription,
(2) propensity matched for receipt of any prescription medication over the year preceding the index date to better account for patients’ potential preference to receive pharmacological care [41] and
(3) examined for the likelihood of prescription of a negative control outcome medication [42] (any gastrointestinal medication) over the follow-up year to further explore patients’ potential preferences towards pharmacological care, with the latter two changes replacing our previous E-value sensitivity analysis.
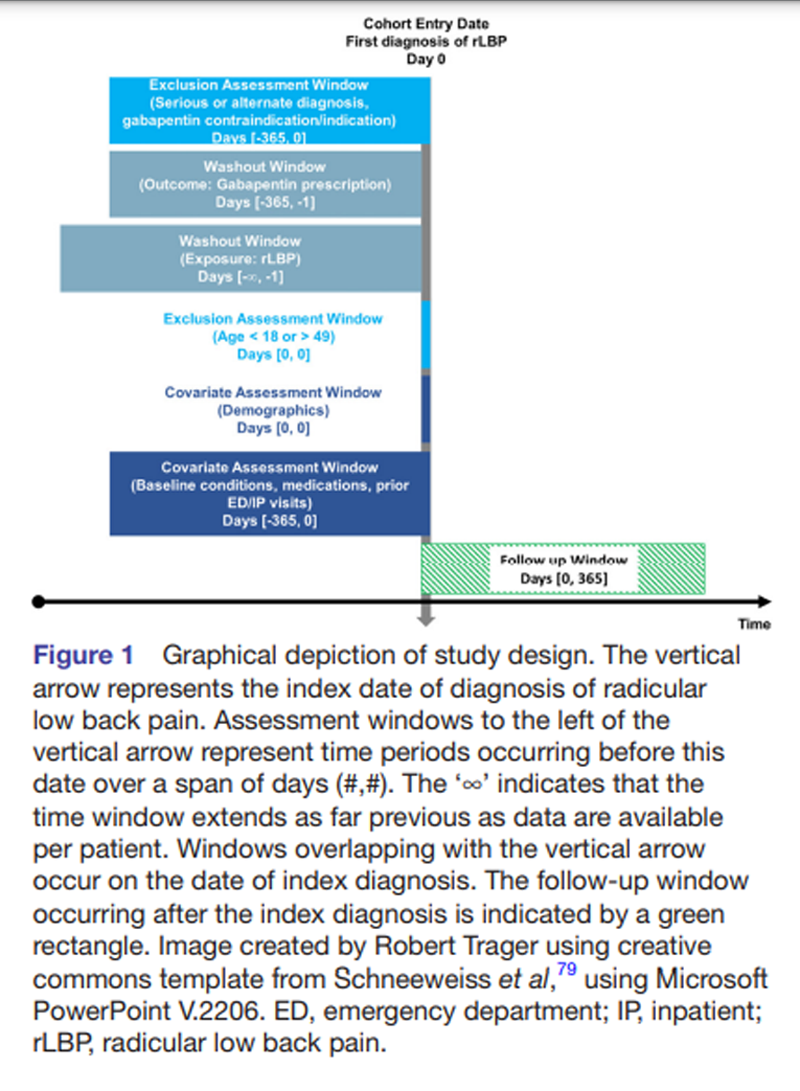
Figure 1 As practice guidelines and prescribing patterns for gabapentin have evolved over time, only data from the most recent 5–year span were included (15 June 2017 to 15 June 2023). To allow for a 1–year follow-up for included patients, only patients with an index diagnosis of rLBP up to 1–year preceding the query date (15 June 2023) were included (enrolment ending 15 June 2022). To help ensure patients were not lost to follow-up, patients were required to have at least one additional healthcare encounter of any kind during the year following the index date of rLBP diagnosis (Figure 1).
Setting and data source
Study data were sourced from a US research network (TriNetX, Cambridge, Massachusetts, USA), [43] which includes aggregated, de-identified data from linked electronic medical records, medical claims and pharmacy claims of 122 million patients. This network includes 84 large academically affiliated healthcare organisations and their outpatient, inpatient and specialty offices, which remain anonymous per data use agreement. The database is searched using standard terminology, such as the International Classification of Disease (ICD) codes. A centralised TriNetX team routinely assesses the data set for completeness, conformance and plausibility. [43, 44] A prior study estimated that medication data was at least 87% complete in the TriNetX data set. [45] At University Hospitals, access to the TriNetX network is managed by Clinical Research Center personnel.
Data regarding the characteristics of chiropractors in the included study sites also remain anonymous. However, chiropractors in integrated healthcare organisations are typically employed within physical medicine and rehabilitation or physical therapy offices, and have on average 21 years of clinical experience with over 6 years working in the integrated care setting. [46]
ParticipantsEligibility criteria
Inclusions
Patients aged 18–49 years were included at the first occurring (index) date of rLBP diagnosis. Only patients with rLBP were included, as this type of LBP often involves neuropathic pain, which is the suggested therapeutic target for gabapentin. [7] The washout period for rLBP extended as far as data were available preceding the index diagnosis date (which varied per patient), such that patients had no prior recorded diagnosis of rLBP. The current study definition for rLBP included ICD codes that describe sciatica and lumbosacral radiculopathy (online supplemental table 1). [47] This definition did not include diagnoses related to disc degeneration, disc herniation and spondylosis, which may cause axial LBP without radiculopathy. [48]
Neuropathic pain is more common in those with LBP related to lumbar disc herniation compared with lumbar stenosis, scoliosis or spondylolisthesis. [49] The age bracket of adults under 50 was selected as rLBP is more likely to result from lumbar disc herniation in patients of this age, [50–52] while older patients are more likely to have lumbar stenosis underlying rLBP. [53] Focusing on a narrower population with rLBP in the current study aimed to create a participant pool with more homogeneous acute pathophysiology, as the likelihood of neuropathic pain (ie, the therapeutic target of gabapentin) varies across LBP aetiologies. [49]
Patients were divided into two cohorts based on receipt of CSMT versus usual medical care. The CSMT cohort served as the test cohort, while the cohort receiving usual medical care served as an active comparator. Patients receiving CSMT on the date of index diagnosis of rLBP were included in the CSMT cohort, while patients not receiving CSMT on the date of index diagnosis formed the cohort receiving usual medical care (online supplemental table 2). In the USA, treatment codes describing CSMT are used almost exclusively by chiropractors. [54] Usual medical care was defined for the purposes of this study as any of a range of medical services besides CSMT, including physical therapy, medications and interventional or surgical procedures.
Exclusions
Our case definition for rLBP excluded patients with serious pathology such as malignancy, fracture, infection and cauda equina syndrome, in accordance with prior, similar studies (online supplemental table 3). [32, 34, 55, 56] In addition, those with previous lumbar surgery, scoliosis, spondylolisthesis, lumbosacral plexopathy, myelopathy, fibromyalgia and multiple sclerosis were excluded, as these conditions represent alternate causes or mimickers of rLBP [57, 58] and may have a different treatment approach with regards to chiropractic care and gabapentin prescription.
Patients with seizure disorders and epilepsy, diabetic neuropathy, herpetic neuralgia and spinal cord injury were broadly excluded as these represent FDA-approved indications for gabapentin in the USA. [59, 60] Similarly, patients with restless leg syndrome were excluded as this condition represents an FDA-approved indication for gabapentin enacarbil. [59] Those with myasthenia gravis and myoclonus, conditions which represent contraindications to gabapentin prescription, were also excluded. [12] All exclusions were made over the year preceding the index date of rLBP diagnosis (figure 1).
VariablesGabapentin
Gabapentin prescription occurring over a 1–year follow-up window after index rLBP diagnosis was examined using the RxNorm code for gabapentin (25 480). A 1–year follow-up was chosen to account for the natural history of rLBP, which typically improves over a span of 3 months to 1 year. [31, 62] In addition, a 1–year follow-up allowed for comparison to normative data describing the frequency of gabapentin prescription. [21]
As the study design was customised to examine gabapentin alone, it was not possible to examine prescription of other gabapentinoids or anticonvulsants (eg, pregabalin, topiramate). Prescription of pregabalin was factored into our propensity matching model; thus, it could not be recorded as an individual outcome. In addition, similar antiepileptic medications such as pregabalin are less frequently prescribed for LBP compared with gabapentin [21] and thus may require a larger sample size. Finally, different Controlled Substance Scheduling, [63] use indications, precautions and contraindications would require a different study methodology for each medication.
Potential confounders
Propensity score matching was used to reduce bias [37] by balancing patient characteristics between the CSMT and usual medical care cohorts which had a known relationship to the outcome of interest, odds of gabapentin prescription (online supplemental table 4). [64] Key variables present within 365 days of the index diagnosis of rLBP were propensity matched. Covariates with a positive or negative association with gabapentin use or prescription [63, 65–69] or conditions which are common off-label indications for gabapentin [59, 70] were selected for matching based on the available literature:
Adjuvant analgesic use (positive): [66] antiarrhythmics, antidepressants, benzodiazepines, corticosteroids, muscle relaxants, serotonin-norepinephrine reuptake inhibitors, other anticonvulsants (ie, topiramate, pregabalin), tricyclic antidepressants.
Anxiety, bipolar disorder and depression (positive). [67]
Chronic pain (positive). [66]
Demographics: age, sex and race/ethnicity (positive or negative). [65–67, 71]
Diabetes (positive). [63]
Emergency department or inpatient visit (negative). [67]
Headaches, including migraines (positive). [59, 66, 69]
Insomnia (positive). [67]
Irritable bowel syndrome (positive). [66, 70]
Opioid use (positive). [65, 67]
Smoking status, current or former (positive). [65]
Social determinants: unemployment, problems related to economic circumstances (positive). [65, 66]
Substance use disorder (positive). [63, 67]
This study did not exhaustively propensity match for all off-label uses for gabapentin such as interstitial cystitis, hot flashes, hiccups, essential tremors, refractory chronic cough, nausea and vomiting and pruritus. [70, 72] Evidence suggests that these conditions are either not independently associated with gabapentin use [68] or are uncommon reasons for prescription. [69]Study size
A required total sample size of 515 patients was calculated with G*Power (V.3.1.9.7), using a z-test for logistic regression and assuming normal distribution. Parameters included a power of 0.95, two tails, alpha error of 0.05 and OR of 0.67 from a similar study regarding benzodiazepine prescription and CSMT for rLBP. [33] The probability for the alternative hypothesis was 0.20, reflecting the frequency of gabapentin prescription in patients with LBP in a previous study. [21] This sample appeared feasible given the large CSMT population in our previous similar study also using the TriNetX network. [33]
Statistical methods
Key baseline characteristics included in propensity matching were compared using a Pearson χ2 test for categorical variables and independent-samples t-test for continuous variables. Propensity matching was conducted in real-time using software built into the TriNetX data set viewing platform. Propensity score matching involved 1:1 greedy nearest neighbour matching with a calliper distance of 0.1 pooled SDs of the logit of the propensity score. Odds of gabapentin prescription per cohort were calculated by dividing the number of patients receiving a prescription by the number of patients not receiving a prescription. ORs for gabapentin prescription occurring over a 1–year follow-up were calculated by dividing odds in the CSMT cohort by odds in the cohort receiving usual medical care. We did not perform imputations for missing data.
At the recommendation of peer reviewers, we conducted two post hoc sensitivity analyses. A cumulative incidence graph with 95% CIs was used to illustrate the timing of gabapentin prescription and ascertain if, and when, the incidence curves diverged in relation to the index date of rLBP diagnosis. We also examined the likelihood of a negative control outcome [42] to provide a marker of residual between-cohort imbalance in patient preference towards receiving pharmacological care. This was described in terms of an OR for receipt of any gastrointestinal medication over the 1–year follow-up window, and was calculated using the same methods described above for gabapentin.
Patient and public involvement
No patient or public involvement.
Results
Participants
Eligible patients were identified from 77 healthcare organisations, 10 of which included CSMT as an offered service. Before propensity matching, there were 1,635 patients in the CSMT cohort and 429,778 in the cohort receiving usual medical care. During propensity matching the larger usual medical care cohort diminished in size as patients that did not match were removed, resulting in 1,635 patients in each cohort (mean age 36.3±8.6 years, 60% women).

Table 1
page 6Before matching, there were several between-cohort differences (Table 1). For example, the CSMT cohort had a significantly greater percentage of patients who were white and not Hispanic/Latino, and lower representation of other racial and ethnic groups. The CSMT cohort had a greater frequency of ‘anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders’, mood disorders and prescription of antidepressants, among other differences. After matching, no variables were significantly different between cohorts (ie, p>0.05 for each).
Descriptive data
The mean number of data points per patient was high in both cohorts (CSMT 1433; usual medical care 989). After propensity matching, the frequency of several ‘unknown’ demographic variables was the same in both cohorts: unknown race (19%) unknown sex (1%), unknown age (0%). Unknown ethnicity was similar in both cohorts (14% CSMT, 15% usual medical care). Together, these findings suggested there were inconsequential between-cohort differences regarding missing data. A density graph of propensity scores revealed that cohorts were similar after matching (online supplemental figure 1).
Key results

Table 2 Gabapentin prescription was less frequent in the CSMT cohort over the 1–year follow-up after rLBP diagnosis both before and after propensity matching. After matching, 4.6% of patients in the CSMT cohort and 8.3% in the usual medical care cohort had received a gabapentin prescription (Table 2). After matching, odds of gabapentin prescription over the 1–year follow-up were significantly lower in the CSMT cohort compared with the cohort receiving usual medical care, with an OR (95% CI) of 0.53 (0.40 to 0.71; p<0.0001).
Sensitivity analyses

Figure 2 Analysis of the cumulative incidence graph revealed that the incidence of gabapentin prescription was greater in the usual medical care cohort than the CSMT cohort at day 0 (Figure 2). The incidence of gabapentin prescription remained higher in the usual medical care cohort for the duration of follow-up, and the incidence curves and 95% CIs did not overlap at any point during follow-up, suggesting that the incidence was significantly different between cohorts throughout.
After propensity score matching, there was no significant difference in the likelihood of receiving any gastrointestinal medication over 1–year follow-up in the CSMT cohort compared with the usual care cohort (OR 0.89 (0.76–1.04)) with an incidence of 26% (CSMT) and 28% (usual medical care).
Discussion
To our knowledge, this retrospective cohort study was the first to examine the association between CSMT and the likelihood of gabapentin prescription among patients with rLBP and included a large sample size with over 1,600 patients per propensity matched cohort. These real-world findings support our hypothesis that adults initially receiving CSMT for rLBP have reduced odds of receiving a gabapentin prescription over a 1–year follow-up period. Our cumulative incidence analysis suggested that much of the difference in likelihood in prescription could be attributed to the care received on the date of diagnosis of rLBP, either being pharmacological (usual medical care) or non-pharmacological (CSMT). Per a negative control outcome, our results were not explained by a patient preference to avoid prescription medications.
In a previous study based on 2,018 survey data, 20% of US adults (mean age 52) who visited a medical doctor for LBP over the preceding year were recommended gabapentin. [21] In comparison, the present study found that only 8% of the usual medical care cohort received a gabapentin prescription.
The comparatively lower rate of gabapentin prescription in our study may be due several differences in study design such as:(1) our rigorous selection criteria excluded several conditions positively associated with gabapentin prescription (eg, diabetic neuropathy, restless legs syndrome);
(2) our new-user design led to the inclusion of younger patients earlier in their course of care; and
(3) our study measured documented prescriptions, rather than recommendation of the medication based on patients’ recollection.Our findings are similar to those of previous studies which demonstrated an association between initial receipt of CSMT and reduced odds of prescription of opioids and benzodiazepines. [33, 35, 36] Gabapentin, opioids and benzodiazepines are similarly not recommended by several clinical practice guidelines for acute LBP/rLBP. [13] Accordingly, our findings add to growing evidence that receipt of CSMT early in the care pathway for new onset LBP/rLBP could lead to greater concordance with these guidelines with respect to medication prescribing practices. [33, 35, 36] In addition, our findings are consistent with some authors’ recommendations that patients with LBP/rLBP should initiate treatment with non-pharmacological providers such as chiropractors. [19, 73]
There are several potential explanations as to why initial CSMT for rLBP could be associated with a reduction in gabapentin prescription. First, while US chiropractors are portal-of-entry providers, they do not prescribe medications, including gabapentin. As such, they are not faced with pressure or even the option to prescribe medications for pain. In addition, rLBP generally has a good prognosis, with most patients improving by 1 year. [61, 62]
Therefore, we suspect that patients visiting a chiropractor initially for rLBP(1) may improve with CSMT,
(2) improve via the favourable natural history of rLBP or
(3) enter a non-pharmacological care pathway instead of visiting providers who have medication prescription as part of their scope of practice, and thus be more likely to prescribe gabapentin.Considering that previous randomised controlled trials have found that CSMT is effective in alleviating LBP [74] and rLBP, [75, 76] it remains possible that pain relief afforded by CSMT accounts for the observed reduction in gabapentin prescription. However, we are unaware of any studies that examined gabapentin prescription alongside markers of pain and/or disability that could further support this hypothesis. Accordingly, a future pragmatic clinical trial could examine the potential interaction between pain relief and likelihood of gabapentin prescription among patients randomised to enter a chiropractic or medical care pathway for new onset rLBP.
The reduction in absolute risk of gabapentin prescription over 1–year follow-up was relatively small in the present study (4%). However, we cannot rule out a clinically important effect considering the potential risk of abuse, misuse, dependence, withdrawa [19] and adverse events [12] related to gabapentin use. One previous study found that patients who received CSMT for LBP had significantly reduced odds of having an adverse drug reaction (OR of 0.49), [77] suggesting that reduced prescription of medications used to treat pain could translate into less adverse events. However, we were unable to examine for the likelihood of potential adverse events related to gabapentin in our study considering:(1) we had limited sample size to evaluate this outcome,
(2) our study population was highly selected, via excluding or controlling for comorbid conditions and potential drug interactions and
(3) data regarding the dose of gabapentin was unavailable.A follow-up study, if sufficiently powered with a larger data range and data regarding dose, could better examine markers related to clinical significance (eg, adverse events).
Similar retrospective cohort studies should be undertaken to further explore the association between CSMT and gabapentin prescription using other large data sets which may include different patient populations (eg, Medicare, Medicaid) or healthcare settings (eg, Veterans Health Administration, private practices or practice-based research networks). Similar results to the current study would then justify a prospective study, such as a randomised controlled trial, to reduce residual sources of confounding. A prospective trial would also allow for related health outcomes such as changes in health-related quality of life, pain severity, additional social determinants and LBP-related direct or indirect costs to be examined in tandem.
Limitations
This study has several limitations. First, there may be unmeasured confounding. Despite our efforts to control for socioeconomic variables relating to income and education level, these variables may not have been sufficiently represented in the TriNetX data set. Other variables which may influence gabapentin prescription, such as geographical location, [65] pain severity and LBP-related disability, were unavailable in the data set.
Second, patients could be misclassified. As the study included data derived from medical records, diagnoses or comorbidities could be missing, outdated or incorrect. Metrics regarding data completeness were unavailable for several variables. Our query could not be validated against a gold-standard chart review given that data were de-identified and aggregated from several sources.
Third, patients’ eligibility could change during follow-up. For example, patients could have received a diagnosis of diabetic neuropathy after rLBP diagnosis, which was not present at baseline. While this could not be completely prevented, we minimised the potential for between-cohort differences in changing eligibility by the extensive use of propensity score matching at baseline (eg, matching for diabetes mellitus).
Fourth, we were unable to compare gabapentin prescribing rates according to initial provider type as the TriNetX data set does not catalogue provider codes. As rates of gabapentin prescribing may vary across provider type, [65] this information would allow for a more in-depth analysis. In addition, based on previous literature regarding opioids, [19, 34, 36] it is possible that initiating care for rLBP with any non-pharmacological provider (ie, physical therapist, acupuncturist, chiropractor) would similarly yield a reduction in prescribing of gabapentin.
Fifth, this study did not incorporate non-clinical factors such as a pressure to prescribe medications for pain, patients’ expectations or providers’ concern regarding patient satisfaction surveys, which could influence the likelihood of gabapentin prescription. [78]
Sixth, this study did not examine markers of gabapentin misuse, abuse or illicit use, which are not adequately recorded in the data set. However, our strategy of propensity matching for substance use disorders aimed to minimise confounding related to this possibility.
Seventh, gabapentin prescriptions were temporally but not deterministically linked to rLBP diagnoses; therefore, it remains possible that their prescription may have been for another condition. This possibility was minimised by our strategy to exclude patients with potential on-label gabapentin indications (eg, seizure disorders, diabetic neuropathy), and account for patients with potential off-label uses of gabapentin via exclusion (eg, fibromyalgia) [59, 70] or propensity matching (eg, anxiety, irritable bowel syndrome). [66, 70]
Finally, study results may only be generalisable to large academic healthcare organisations and may not apply to smaller private practice settings. Further, study results may not be generalisable to healthcare settings outside of the USA, which may have varied legal status and guideline recommendations regarding the prescription of gabapentin for rLBP.
Conclusion
This large retrospective cohort study found that adults receiving CSMT for a new diagnosis of rLBP have significantly reduced odds of receiving a gabapentin prescription over 1–year follow-up compared with those receiving usual medical care. According to our sensitivity analyses, the difference in incidence of prescription was largely attributed to the type of care received on the index date of rLBP diagnosis, and was not explained by a preference for patients in the CSMT cohort to avoid prescription medications. These findings are consistent with a potential influence of early CSMT on patients’ rLBP care pathway towards avoiding certain prescription medications. However, our findings may not be generalisable to smaller practice settings or other countries and should be replicated and corroborated by a prospective study to reduce residual sources of confounding.
Supplementary Material
Supplemental file Supplemental Tables and Figure
Acknowledgements
This publication was made possible through the support of the Clinical Research Center of University Hospitals Cleveland Medical Center (UHCMC) and the Case Western Reserve University Clinical and Translational Science Collaborative (CTSC) 4UL1TR000439. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of UHCMC or National Institutes of Health.
Contributors
RJT, ZAC, RS, RMC, JAP and JAD conceived of and designed the study protocol and methodology.
RMC and JAP directly accessed the study software and data set and performed data collection.
RJT, ZAC, JAP and JAD formally analysed and interpreted data. JAD provided supervision and mentorship.
RJT drafted the initial manuscript, while all authors contributed to, critically revised and approved of the final manuscript.
RJT was the guarantor of the study.
Disclaimer
The views expressed are those of the authors and do not necessarily reflect the official policy or position of the US Department of Veterans Affairs or the US Government.
References:
Chen S, Chen M, Wu X, Lin S, Tao C, Cao H, et al.
Global, Regional and National Burden of Low Back Pain
1990-2019: A Systematic Analysis of the Global
Burden of Disease Study 2019
J Orthop Translat 2021 (Sep 10); 32: 49–58Dieleman JL, Cao J, Chapin A, et al.
US Health Care Spending by Payer and Health Condition, 1996-2016
JAMA 2020 (Mar 3); 323 (9): 863–884Vining RD, Minkalis AL, Shannon ZK, et al.
Development of an Evidence-Based Practical Diagnostic
Checklist and Corresponding Clinical Exam for Low Back Pain
J Manipulative Physiol Ther. 2019 (Nov); 42 (9): 665–676Vulfsons S, Bar N, Eisenberg E.
Back pain with leg pain.
Curr Pain Headache Rep 2017;21:32.
doi:10.1007/s11916-017-0632-xMorlion B.
Pharmacotherapy of low back pain:
targeting nociceptive and neuropathic pain components.
Curr Med Res Opin 2011;27:11–33.
doi:10.1185/03007995.2010.534446Kukkar A, Bali A, Singh N, et al.
Implications and mechanism of action of gabapentin in neuropathic pain.
Arch Pharm Res 2013;36:237–51.
doi:10.1007/s12272-013-0057-yRosenquist RW.
Gabapentin.
J Am Acad Orthop Surg 2002;10:153–6.
doi:10.5435/00124635-200205000-00001Mellegers MA, Furlan AD, Mailis A.
Gabapentin for neuropathic pain:
systematic review of controlled and uncontrolled literature.
Clin J Pain 2001;17:284–95.
doi:10.1097/00002508-200112000-00002Evoy KE, Sadrameli S, Contreras J, et al.
Abuse and misuse of pregabalin and gabapentin:
a systematic review update.
Drugs 2021;81:615–7.
doi:10.1007/s40265-021-01495-0Giménez-Campos MS, Pimenta-Fermisson-Ramos P, Díaz-Cambronero JI, et al.
A systematic review and meta-analysis of the effectiveness and
adverse events of gabapentin and pregabalin for sciatica pain.
Aten Primaria 2022;54:102144.
doi:10.1016/j.aprim.2021.102144Enke O, New HA, New CH, et al.
Anticonvulsants in the treatment of low back pain and
lumbar radicular pain: a systematic review and meta-analysis.
CMAJ 2018;190:E786–93.
doi:10.1503/cmaj.171333Quintero GC.
Review about gabapentin misuse, interactions, contraindications and side effects.
J Exp Pharmacol 2017;9:13–21.
doi:10.2147/JEP.S124391Price MR, Cupler ZA, Hawk C, et al.
Systematic review of guideline-recommended medications
prescribed for treatment of low back pain.
Chiropr Man Therap 2022;30:26.
doi:10.1186/s12998-022-00435-3Qaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Kreiner DS, Matz P, Bono CM, et al.
Guideline summary review: an evidence-based clinical guideline
for the diagnosis and treatment of low back pain.
Spine J 2020;20:998–1024.
doi:10.1016/j.spinee.2020.04.006Chou R, Cote P, Randhawa K, Torres P, Yu H, Nordin M et al (2017)
The Global Spine Care Initiative: Applying Evidence-based Guidelines
on the Non-invasive Management of Back and Neck Pain to Low-
and Middle-income Communities
European Spine Journal 2018 (Sep); 27 (Suppl 6): 851–860Pangarkar SS, Kang DG, Sandbrink F, et al.
VA/Dod clinical practice guideline:
diagnosis and treatment of low back pain.
J Gen Intern Med 2019;34:2620–9.
doi:10.1007/s11606-019-05086-4Department of Veterans Affairs / Department of Defense / U.S.
VA/DoD Clinical Practice Guideline for the Diagnosis
and Treatment of Low Back Pain
Government Printing Office, 2022 (141 pages).Buchbinder R, Underwood M, Hartvigsen J, Maher CG (2020)
The Lancet Series Call to Action to Reduce Low Value Care
for Low Back Pain: An Update
Pain. 2020 (Sep); 161 (1): S57–S64Morgan DJ, Dhruva SS, Coon ER, et al.
Update on medical overuse.
JAMA Intern Med 2019;179:240–6.
doi:10.1001/jamainternmed.2018.5748Goertz CM, Long CR, English C, et al.
Patient-reported physician treatment recommendations and
compliance among US adults with low back pain.
J Altern Complement Med 2021;27:S99–105.
doi:10.1089/acm.2020.0392Costales B, Brown JD, Goodin AJ. Pdg46
outpatient off-label gabapentin utilization from 2011 to 2015
among United States adults.
Value in Health 2020;23:S137.
doi:10.1016/j.jval.2020.04.334Peet ED, Dana B, Sheng FY, et al.
Trends in the concurrent prescription of opioids and
gabapentin in the US, 2006 to 2018.
JAMA Intern Med 2023;183:162–4.
doi:10.1001/jamainternmed.2022.5268Chang M.
The Chiropractic Scope of Practice in the United States:
A Cross-sectional Survey
J Manipulative Physiol Ther. 2014 (Jul); 37 (6): 363–376Beliveau PJH, Wong JJ, Sutton DA, Simon NB, Bussieres AE, Mior SA, et al.
The Chiropractic Profession: A Scoping Review of Utilization Rates,
Reasons for Seeking Care, Patient Profiles, and Care Provided
Chiropractic & Manual Therapies 2017 (Nov 22); 25: 35National Board of Chiropractic Examiners:
Practice Analysis of Chiropractic 2020
NBCE, 2020.Hurwitz EL:
Epidemiology: Spinal Manipulation Utilization
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 648–654Lewis RA, Williams NH, Sutton AJ, et al.
Comparative clinical effectiveness of management strategies
for sciatica: systematic review and network meta-analyses.
Spine J 2015;15:1461–77.
doi:10.1016/j.spinee.2013.08.049Rubinstein SM, de Zoete A, van Middelkoop M, et al.
Benefits and Harms of Spinal Manipulative Therapy for the Treatment
of Chronic Low Back Pain: Systematic Review and Meta-analysis
of Randomised Controlled Trials
British Medical Journal 2019 (Mar 13); 364: l689Van Wambeke P, Desomer A, Ailiet L.
Low Back Pain and Radicular Pain:
Assessment and Management 2017
Belgian Health Care Knowledge Centre KCE Report 287CsBernstein IA, Malik Q, Carville S, et al.
Low back pain and sciatica: summary of NICE guidance.
BMJ 2017;356:i6748.
doi:10.1136/bmj.i6748Fritz JM, Kim J, Dorius J.
Importance of the Type of Provider Seen to Begin Health Care
for a New Episode Low Back Pain: Associations
with Future Utilization and Costs
J Eval Clin Pract. 2016 (Apr); 22 (2): 247–252Trager RJ, Cupler ZA, DeLano KJ, et al.
Association Between Chiropractic Spinal Manipulation
and Gabapentin Prescription in Adults With Radicular
Low Back Pain: Retrospective Cohort Study Using US Data
BMJ Open 2023 (Jul 21); 13 (7): e073258Kazis LE, Ameli O, Rothendler J, et al.
Observational Retrospective Study of the Association of
Initial Healthcare Provider for New-onset Low Back Pain
with Early and Long-term Opioid Use
BMJ Open. 2019 (Sep 20); 9 (9): e028633Corcoran KL, Bastian LA, Gunderson CG, et al.
Association Between Chiropractic Use and Opioid Receipt Among
Patients with Spinal Pain: A Systematic Review and Meta-analysis
Pain Medicine 2020 (Feb 1); 21 (2): e139–e145Harwood KJ, Pines JM, Andrilla CHA, et al.
Where to Start? A Two Stage Residual Inclusion Approach
to Estimating Influence of the Initial Provider
on Health Care Utilization and Costs for
Low Back Pain in the US
BMC Health Serv Res 2022 (May 23); 22 (1): 694Gokhale M, Stürmer T, Buse JB.
Real-world evidence: the devil is in the detail.
Diabetologia 2020;63:1694–705.
doi:10.1007/s00125-020-05217-1Franklin JM, Schneeweiss S.
When and how can real world data analyses substitute
for randomized controlled trials.
Clin Pharmacol Ther 2017;102:924–33.
doi:10.1002/cpt.857Trager RJ, Daniels CJ, Perez JA, et al.
Association Between Chiropractic Spinal Manipulation and
Lumbar Discectomy in Adults with Lumbar Disc Herniation
and Radiculopathy: Retrospective Cohort Study
Using United States' Data
BMJ Open 2022 (Dec 16); 12 (12): e068262von Elm E, Altman DG, Egger M, et al.
The strengthening the reporting of observational studies in epidemiology
(STROBE) statement: guidelines for reporting observational studies.
Ann Intern Med 2007;147:573–7.
doi:10.7326/0003-4819-147-8-200710160-00010Sharma R, Haas M, Stano M.
Patient Attitudes, Insurance, and Other Determinants of
Self-referral to Medical and Chiropractic Physicians
American J Public Health 2003 (Dec); 93 (12): 2111–2117Lipsitch M, Tchetgen Tchetgen E, Cohen T.
Negative controls: a tool for detecting confounding and bias
in observational studies.
Epidemiology 2010;21:383–8.
doi:10.1097/EDE.0b013e3181d61eebTopaloglu U, Palchuk MB.
Using a federated network of real-world data to optimize clinical trials operations.
JCO Clin Cancer Inform 2018;2:1–10.
doi:10.1200/CCI.17.00067Pfaff ER, Girvin AT, Gabriel DL, et al.
Synergies between centralized and federated approaches to data quality:
a report from the National COVID cohort collaborative.
J Am Med Inform Assoc 2022;29:609–18.
doi:10.1093/jamia/ocab217Evans L, London JW, Palchuk MB.
Assessing real-world medication data completeness.
J Biomed Inform 2021;119:103847.
doi:10.1016/j.jbi.2021.103847Salsbury, SA, Goertz, CM, Twist, EJ, and Lisi, AJ.
Integration of Doctors of Chiropractic Into Private Sector
Health Care Facilities in the United States:
A Descriptive Survey
J Manipulative Physiol Ther. 2018 (Feb); 41 (2): 149–155Stynes S, Konstantinou K, Dunn KM.
Classification of patients with low back-related leg pain:
a systematic review.
BMC Musculoskelet Disord 2016;17:226.
doi:10.1186/s12891-016-1074-zSuri P, Boyko EJ, Goldberg J, et al.
Longitudinal associations between incident lumbar spine MRI findings
and chronic low back pain or radicular symptoms: retrospective analysis
of data from the longitudinal assessment of imaging
and disability of the back (LAIDBACK).
BMC Musculoskelet Disord 2014;15:152.
doi:10.1186/1471-2474-15-152Orita S, Yamashita T, Ohtori S, et al.
Prevalence and location of neuropathic pain in lumbar spinal disorders:
analysis of 1804 consecutive patients with primary lower back pain.
Spine (Phila Pa 1976) 2016;41:1224–31.
doi:10.1097/BRS.0000000000001553Konstantinou K, Dunn KM, Ogollah R, et al.
Characteristics of Patients with Low Back and Leg Pain
Seeking Treatment in Primary Care: Baseline Results
from the ATLAS Cohort Study
BMC Musculoskelet Disord. 2015 (Nov 4); 16: 332Hernández CP, Sánchez N, Navarro-Siguero A, et al.
What are the causes of sciatica and Radicular pain?
In: Laroche F, Perrot S, eds.
Managing sciatica and radicular pain in primary care practice.
Springer Healthcare Ltd, 2013: 17–31.
doi:10.1007/978-1-907673-56-6_2Jönsson B, Strömqvist B.
Influence of age on symptoms and signs in lumbar disc herniation.
Eur Spine J 1995;4:202–5. doi:10.1007/BF00303410Miyakoshi N, Hongo M, Kasukawa Y, et al.
Prevalence, spinal alignment, and mobility of lumbar spinal stenosis
with or without chronic low back pain: a community-dwelling study.
Pain Res Treat 2011;2011:340629.
doi:10.1155/2011/340629Whedon JM, Haldeman S, Petersen CL, et al.
Temporal trends and geographic variations in the supply of clinicians
who provide spinal manipulation to medicare beneficiaries:
a serial cross-sectional study.
J Manipulative Physiol Ther 2021;44:177–85.
doi:10.1016/j.jmpt.2021.02.002Cherkin DC, Deyo RA, Volinn E, et al.
Use of the International classification of diseases (ICD-9-CM) to identify
hospitalizations for mechanical low back problems in administrative databases.
Spine 1992;17:817–25.
doi:10.1097/00007632-199207000-00015Chu EC-P, Trager RJ.
Prevalence of serious pathology among adults with low back pain
presenting for chiropractic care: a retrospective chart review
of integrated clinics in Hong Kong.
Med Sci Monit 2022;28:e938042.
doi:10.12659/MSM.938042Urits I, Burshtein A, Sharma M, et al.
Low back pain, a comprehensive review:
pathophysiology, diagnosis, and treatment.
Curr Pain Headache Rep 2019;23:23.
doi:10.1007/s11916-019-0757-1Louw J.
The differential diagnosis of neurogenic and referred leg pain.
SA Orthopaedic Journal 2014;13:52–6.Wallach JD, Ross J.
Off-label use, and lessons for postmarketing evaluation efforts.
JAMA 2018;319:776–8.
doi:10.1001/jama.2017.21897Goodman CW, Brett AS.
A clinical overview of off-label use of gabapentinoid drugs.
JAMA Intern Med 2019;179:695–701.
doi:10.1001/jamainternmed.2019.0086Haugen AJ, Grřvle L, Brox JI, et al.
Estimates of success in patients with sciatica due to
lumbar disc herniation depend upon outcome measure.
Eur Spine J 2011;20:1669–75.
doi:10.1007/s00586-011-1809-3Vroomen PCAJ, de Krom MCTFM, Knottnerus JA.
Predicting the outcome of sciatica at short-term follow-up.
Br J Gen Pract 2002;52:119–23.Pauly NJ, Delcher C, Slavova S, et al.
Trends in gabapentin prescribing in a commercially insured
U.S. adult population, 2009-2016.
J Manag Care Spec Pharm 2020;26:246–52.
doi:10.18553/jmcp.2020.26.3.246Bergstra SA, Sepriano A, Ramiro S, et al.
Three handy tips and a practical guide to improve your propensity score models.
RMD Open 2019;5:e000953.
doi:10.1136/rmdopen-2019-000953Zhou L, Bhattacharjee S, Kwoh CK, et al.
Trends, patient and prescriber characteristics in gabapentinoid use
in a sample of United States ambulatory care visits from 2003 to 2016.
J Clin Med 2019;9:83.
doi:10.3390/jcm9010083Zhao D, Baek J, Hume AL, et al.
Geographic variation in the use of gabapentinoids and opioids for pain
in a commercially insured adult population in the United States.
J Pain Res 2022;15:443–54.
doi:10.2147/JPR.S345521Peckham AM, Evoy KE, Covvey JR, et al.
Predictors of gabapentin overuse with or without
concomitant opioids in a commercially insured US population.
Pharmacotherapy 2018;38:436–43.
doi:10.1002/phar.2096Zhao D, Nunes AP, Baek J, et al.
An algorithm to identify gabapentin misuse and/or abuse
in administrative claims data.
Drug Alcohol Depend 2022;235:109429.
doi:10.1016/j.drugalcdep.2022.109429Hamer AM, Haxby DG, McFarland BH, et al.
Gabapentin use in a managed medicaid population.
J Manag Care Pharm 2002;8:266–71.
doi:10.18553/jmcp.2002.8.4.266Yasaei R, Katta S, Saadabadi A.
Gabapentin.
In: StatPearls. StatPearls Publishing, 2022.Ibiloye EA, Barner JC, Lawson KA, et al.
Prevalence of and factors associated with gabapentinoid use
and misuse among Texas Medicaid recipients.
Clin Drug Investig 2021;41:245–53.
doi:10.1007/s40261-021-01009-6Yan PZ, Butler PM, Kurowski D, et al.
Beyond neuropathic pain: gabapentin use in
cancer pain and perioperative pain.
Clin J Pain 2014;30:613–29.
doi:10.1097/AJP.0000000000000014George SZ, Goertz C, Hastings SN, Fritz JM.
Transforming Low Back Pain Care Delivery in the United States
Pain 2020 (Dec); 161 (12): 2667-2673Hurwitz EL, Morgenstern H, Kominski GF, Yu F, Chiang LM.
A Randomized Trial of Chiropractic and Medical Care for Patients with
Low Back Pain: Eighteen-month Follow-up Outcomes from the
UCLA Low Back Pain Study
Spine (Phila Pa 1976). 2006 (Mar 15); 31 (6): 611–621Santilli V, Beghi E, Finucci S.
Chiropractic Manipulation in the Treatment of Acute Back Pain and Sciatica
with Disc Protrusion: A Randomized Double-blind Clinical Trial
of Active and Simulated Spinal Manipulations
Spine J. 2006 (Mar); 6 (2): 131—137McMorland G, Suter E, Casha S, du Plessis SJ, Hurlbert RJ.
Manipulation or Microdiskectomy for Sciatica?
A Prospective Randomized Clinical Study
J Manipulative Physiol Ther. 2010 (Oct); 33 (8): 576–584Whedon JM, Toler AWJ, Goehl JM, et al.
Association between utilization of chiropractic services for
treatment of low back pain and risk of adverse drug events.
J Manipulative Physiol Ther 2018;41:383–8.
doi:10.1016/j.jmpt.2018.01.004Goodman CW, Brett AS.
Gabapentin and Pregabalin for pain —
is increased prescribing a cause for concern
N Engl J Med 2017;377:411–4.
doi:10.1056/NEJMp1704633Schneeweiss S, Rassen JA, Brown JS, et al.
Graphical depiction of longitudinal study designs in health care databases.
Ann Intern Med 2019;170:398–406.
doi:10.7326/M18-3079
Return to LOW BACK PAIN
Return to INITIAL PROVIDER/FIRST CONTACT
Since 7-22-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |