

A Complex Dietary Supplement Extends Longevity of Mice This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Gerontol A Biol Sci Med Sci. 2005 (Mar); 60 (3): 275279 ~ FULL TEXT
Lemon JA, Boreham DR, Rollo CD.
Department of Biology,
McMaster University,
1280 Main Street West,
Hamilton, Ontario,
Canada L8S 4K1.
lemonja@mcmaster.ca
Key factors implicated in aging include reactive oxygen species, inflammatory processes, insulin resistance, and mitochondrial dysfunction. All are exaggerated in transgenic growth hormone mice (TGM), which display a syndrome resembling accelerated aging. We formulated a complex dietary supplement containing 31 ingredients known to ameliorate all of the above features. We previously showed that this supplement completely abolished the severe age-related cognitive decline expressed by untreated TGM. Here we report that longevity of both TGM and normal mice is extended by this supplement. Treated TGM showed a 28% increase (p < .00008) in mean longevity. An 11% increase in mean longevity was also significant (p < .002093) for treated normal mice, compared to untreated normal mice. These data support the hypothesis that TGM are a model of accelerated aging, and demonstrate that complex dietary supplements may be effective in ameliorating aging or age-related pathologies where simpler formulations have generally failed.
From the FULL TEXT Article:
Introduction
Overexpression of growth hormone (GH) in transgenic GH mice (TGM) increases growth rates and adult body sizes twofold over normal mice. All strains of mice overexpressing GH have reduced life spans. [17] For numerous species, including mice, rats, domesticated animals, and humans, body size is negatively correlated to longevity. [710] Longevities of both dwarf mice (disrupted GH axis) and TGM were well described by the regression line from an extensive meta-analysis. [7] Evident pathologies have introduced controversy as to whether TGM express accelerated aging, but many of these pathologies resemble those seen in normal senescent mice, supporting our hypothesis. [6] Regardless, TGM are at least a model of elevated free radical processes in all tissues examined. [3, 11]
Although some studies suggest that GH treatment can offset symptoms of senescence in aging animals [12, 13], others suggest an elevated GH axis may accelerate underlying aging processes. [4, 6] The latter is supported by numerous features of TGM including reduced life span (50% of normal mice), accelerated age-related declines in learning and memory [11, 14], reduced replicative potential of cells in vitro [15], early-onset arthritis [16], elevated endogenous free radical processes that increase with age [3, 5, 17], elevated inflammatory processes [18], and abnormal mitochondria in aging heart. [19] Although mortality is associated with age-related pathologies, particularly in kidney and liver [6, 17, 20, 21], these tissues express elevated free radical processes in TGM [3], and much of this pathology resembles that arising in aged normal mice. [6, 22, 23]
Altered glucose metabolism and elevated levels of insulin have been proposed as important mechanisms in aging. [24, 25] Insulin resistance and hyperinsulinemia are pronounced features of TGM. [26, 27] Cellular damage accruing from reactive oxygen species is considered a key mechanism of cellular aging [28, 29]. Damage may also induce inflammation and further generation of free radicals by immunocytes and microglia. [30] Elevated levels of free radicals and lipid peroxidation in TGM [3], coupled with reduced activity of some antioxidant systems [17], suggests that oxidative stress contributes to reduced survivorship of TGM. GH overexpression may produce relative energetic stress, resulting from a disproportional allocation of resources to growth. This could compromise energy available for other functions including cellular repair, defense, and replacement processes. [3, 31] Alternatively, TGM may also show increased production of free radicals via membrane-bound NAD(P)H oxidase systems that are activated by GH and insulin-like growth factor-I signalling pathways or altered mitochondrial functioning. [7, 32]
Studies testing the effectiveness of antioxidants and other compounds in ameliorating aging have examined single, or at most a few materials in combination. With some exceptions [3339] ), effectiveness has been meager. [40, 41] A prominent group of gerontologists recently underscored that there is no scientific evidence to support the effectiveness of any known anti-aging formulation. [42] We developed a complex dietary supplement comprising 31 ingredients established to reduce oxidative stress and inflammation, promote membrane and mitochondrial integrity, or increase insulin sensitivity [for ingredients, see [11]]. All of these processes have been implicated in aging. [29, 38, 43, 44]
Young TGM learn a cued 8-arm radial maze twice as fast as did age-matched normal mice [11, 45]; however, TGM display a dramatic age-related decline in learning ability. [11] The dietary supplement abolished this cognitive decline, and treated older mice actually learned better than did younger mice. [11] The supplement also ameliorated other biomarkers of aging (i.e., treated TGM showed increased locomotion, reduced hind-end arthritis, fewer cataracts, and improved coat quality). Here we document a significant longevity extension in TGM fed our dietary supplement. Significant improvements in normal mice suggest that the supplement ameliorates normal aging processes.
METHODS
Animals
Our TGM have metallothionein promoters fused to rat GH structural genes. [46] The rat GH genes are incorporated into one chromosome, chronically elevating plasma GH levels more than 100-fold. The original stock was C57BL/6J male Χ SJL female hybrids, donated by Dr. R. Brinster (School of Veterinary Medicine, University of Pennsylvania, Philadelphia). Breeding heterozygously TGM to normal partners yields offspring of both genotypes with otherwise similar genetic backgrounds. TGM were distinguishable by their larger size by 28 days of age.
General Housing Protocols
Four mice were maintained per cage (27 Χ 12 Χ 15.5 cm), bedded with Betachip (Northeastern Products, Warrensburg, NY). A stainless steel hopper provided food ad libitum (LabDiet 5001; PMI Feeds, St. Louis, MO), and supported a polystyrene water bottle. The housing room maintained a 12-h light/dark photoperiod at 22 ± 3°C. All protocols adhered to Canada Council and institutional regulations on animal care.
Dietary Supplement

Table 1 The dietary supplement was designed to simultaneously ameliorate several processes implicated in aging. The exact formulation was previously published [11], and is outlined in Table 1. Dosages for the mice were formulated based on amounts commonly prescribed to humans. Values were adjusted for the smaller body size of the mice, then, dosages were increased by a factor of 10 based on the higher gram-specific metabolic rate (and consequently faster utilization and turnover) of mice compared to humans. [47] The supplement was prepared fresh daily in liquid form, soaked onto a small piece of bagel, and allowed to dry (dry weight of supplement = 140.3 mg per mouse based on a 35 g mouse). All treated mice received this dose midway through the photoperiod. The bagel bits were rapidly eaten, ensuring mice obtained full and equivalent doses. Supplemented mice were fed this formulation starting at 2 months of age, and were maintained on the supplement for their lifetime.
Selection Criteria
Because we were interested mainly in aging, only mice that died at older ages (≥180 days for TGM and ≥ 400 days for normal mice) or that were killed at endpoints (indicating imminent death) were included in the analysis. Such criteria are unlikely to introduce bias into longevity estimates. [48]
RESULTS

Table 2 Analysis of covariance (Table 2) using body weight as the covariate detected significant impacts of body weight (p <.021585), dietary supplement (p <.0000001), sex (p <.002191), and genotype (p <.0000001) on mean longevity. The only significant interaction effect was between sex and genotype (p <.005954). We previously documented that the supplement had no effect on body weight for sex or genotype. [11] We carried out regression analyses within groups, and found no relationship between body size and longevity within any group studied (data not shown). Although analysis of covariance demonstrated a significant sex effect, we pooled the sexes to avoid small sample sizes and to increase the power of the post hoc tests. It should be noted that 10 TGM were fed plain bagel pieces (i.e., without dietary supplement); however, because there was no difference in life span between treated and untreated TGM, the plain-bagel-fed mice were incorporated into the untreated (i.e., fed only chow) TGM group to increase sample size.

Table 3 Table 3 illustrates that both supplemented TGM and normal mice have a significantly longer mean life span compared to their respective unsupplemented controls. Treated TGM expressed a 28% increase in mean longevity compared to untreated TGM (p <.00008). Even though the sample size for supplemented normal mice is presently small, these mice had an approximately 11% increase in mean longevity compared to control mice (p <.002093). Notably there is no significant difference in body weight for supplemented TGM (p <.765041, df = 323) or supplemented normal mice (p <.968349, df = 98) compared to their respective unsupplemented groups. For complete analysis on differences in body weight between genotypes, see [11].
The longest lived TGM was a supplemented male (677 days). For normal mice, the longest lived mouse was an unsupplemented male (1,153 days). This mouse was unusual, however, in being an isolated virgin. The upper 95% confidence interval (CI) suggests likely extensions in maximal longevity in all supplemented groups (Table 3), but caution is in order because this measure is sensitive to both sample size and variance.
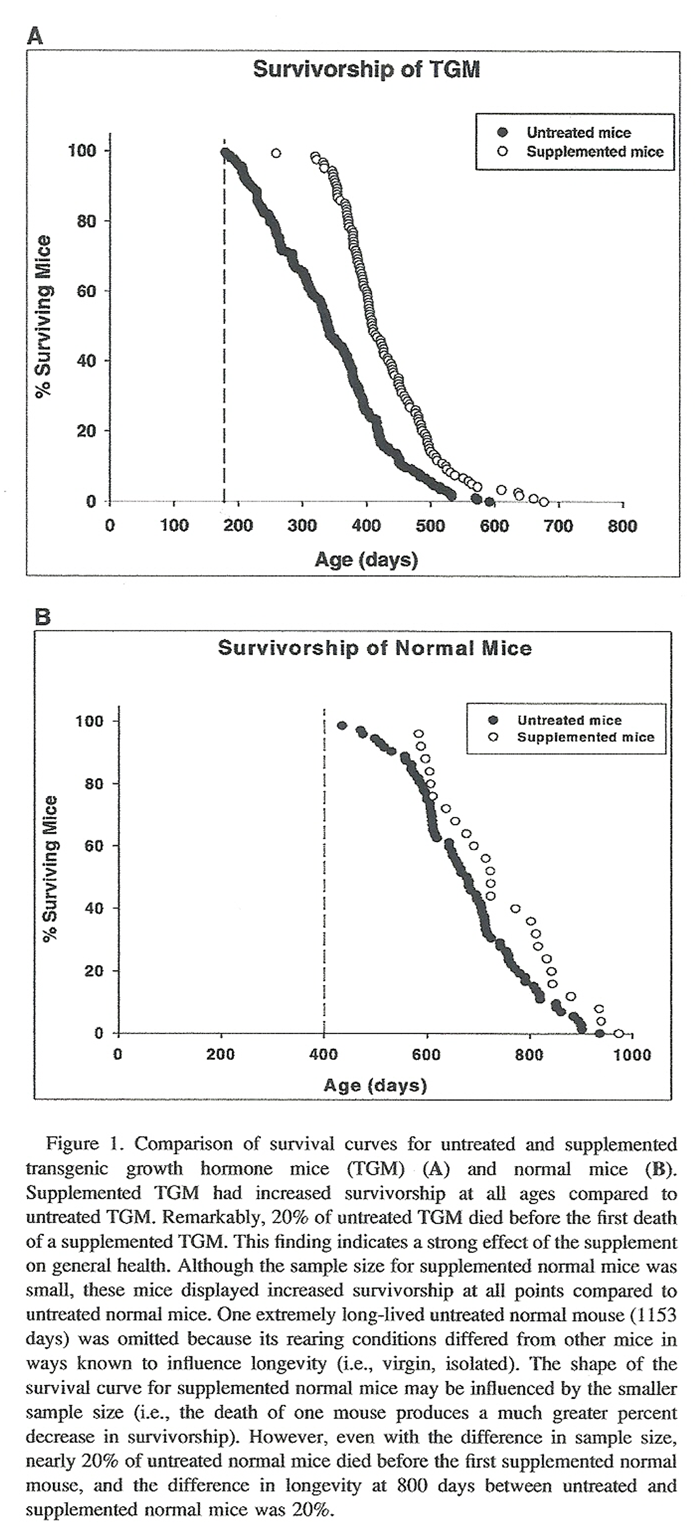
Figure 1 The survival curves for TGM (Figure 1A) illustrate that, compared to untreated TGM, supplemented TGM have significant longevity extension at all points of their life span. The most startling difference was the older age at which the first supplemented TGM died, compared to that of untreated TGM (Figure 1A). The same was true for normal mice (Figure 1B); supplemented normal mice demonstrated a significant longevity extension throughout their life span compared to untreated normal mice. Supplemented mice also showed a dramatic increase in the age of death of the first supplemented normal mouse compared to that of untreated normal mice (Figure 1B). The results suggest positive impacts not only on aging processes, but also on the overall health of supplemented mice.
Visual condition of supplemented 12-month-old TGM compared with that of untreated age-matched TGM was remarkable. Occurrence of cataracts in untreated mice was 6% in normal mice and 14% for TGM. There was no evidence of cataracts in supplemented TGM (p <.0001), and 4% of supplemented normal mice (p >.05, not significant) presented cataracts. Wasting (>20% loss in body weight) occurred in 10.9% and 21.1% of untreated normal mice and TGM, respectively. Wasting was completely absent in supplemented normal mice (p <.001), and was reduced to 13.3% in supplemented TGM (p >.05, not significant). Significant weight loss was usually visible by 8 months and 17 months of age in untreated TGM and normal mice, respectively. Wasting typically occurred no earlier than 12 months of age in supplemented TGM and 22 months of age in supplemented normal mice. Arthritic hindquarters and spines in untreated normal mice occurred in 33.0% and 36.1%, respectively, compared to 4.2% (arthritic hindquarters, p <.0001) and 12.0% (arthritic spines, p <.005) in supplemented normal mice. Arthritis occurred in 63.4% of untreated TGM hindquarters and 59.0% of untreated TGM spines. Arthritic hindquarters and spines were reduced to 8.3% (p <.000005) and 5.0% (p <.000005), respectively, in supplemented TGM.
Although a subjective assessment, coat quality was usually improved in supplemented mice. Thirty-nine percent of untreated normal mice had poor coat quality in senescence, which was reduced to 18.7% in supplemented normal mice (p <.005), whereas 54.0% of unsupplemented TGM had poor coat quality compared to 18.3% of supplemented TGM (p <.0001). A number of supplemented TGM males displayed coats that were at least doubled in thickness compared to those of any other mice. Onset of visible age-related symptoms occurred by 18 months of age, on average, in untreated normal mice and by 21 months of age in supplemented normal mice. The onset of age-related symptoms typically occurred by 6 months of age in untreated TGM, whereas the average age of onset in supplemented TGM was 11 months. The severity of symptoms in both supplemented normal mice and TGM was milder at endpoint compared to that in untreated mice. The superior physical condition of supplemented TGM extends to ages that exceed the longevity of untreated TGM.
Contracted veterinary pathologist reports (McMaster Central Animal Facility) identify pathologies in kidney and liver as main contributors to mortality in untreated TGM and old normal mice, as previously documented by others. [6, 17, 2023] Supplemented TGM show amelioration of brain aging [11], but the endpoint is still associated mainly with age-related degeneration in kidney (membranoproliferative glomerulonephritis) and liver (hepatocellular degeneration). Both conditions were noted by the pathologist as likely caused by free radical disease of a sublethal variety in untreated TGM. Although supplemented TGM sent for pathology were typically 23 months older than untreated TGM, the severity of both liver and kidney pathologies were similar, suggesting that the onset of liver and kidney pathologies may be delayed in supplemented mice. Liver and particularly kidney failure also predominate as cause of death in normal mice; however, the etiology is less severe in normal mice compared to TGM. Extended longevity may arise via amelioration of tissue-specific aging processes and associated development of characteristic age-related pathologies.
DISCUSSION
The success of the use of dietary supplements to ameliorate aging has had meager or inconsistent results. Besides containing many more ingredients than other formulations, many of the ingredients in our supplement (vitamins C, D, E, and glutathione) are strongly synergistic. [35, 39, 40] The targeted mechanisms of aging (i.e., oxidative stress, inflammation, insulin resistance and impaired glucose metabolism, and mitochondrial and membrane deterioration) are largely interdependent. Mitochondrial dysfunction, impaired glucose metabolism, insulin resistance, and inflammatory processes are significant contributors to cellular oxidative stress. Conversely, increased free radical production can exacerbate these processes. Simultaneous treatment for multiple processes contributing to aging may benefit strongly by acting synergistically on these interdependent processes. Our results confirm that complex formulations can indeed obtain remarkable results.
Although maximal longevity is considered to be the best measure for addressing aging, mean and maximal longevities are highly correlated. The mean life span of supplemented TGM and normal mice are significantly extended compared to that of unsupplemented mice. The survival curves for both normal mice and TGM (Figure 1, A and B) indicate that supplemented mice had: (a) significant extension in longevity at all points of their life span and (b) improved health in youth compared to untreated mice. To our knowledge this study provides the first evidence that the early mortality of TGM can be significantly offset by a treatment that also appears to act on the normal aging process.
The finding that the dietary supplement did not have a significant impact on body weight in either TGM or normal mice (Table 3) was unexpected, but this finding eliminates the issue that the longevity extension in supplemented mice could be explained by a change (particularly a reduction) in the body size of the mice. Generally, smaller animals within a species tend to live longer on average than larger ones [7]. Apparently the actions of the supplement work independently of body size to produce the longevity extension in our mice. It will be interesting to determine if reformulation of the dietary supplement to include more effective components (e.g., more effective anti-inflammatories, higher levels of α-lipoic acid to offset liver and kidney stress) will further improve the longevity of both TGM and normal mice.
It is problematic to assess the individual significance of the multitude of physiological factors that influence longevity; however, here we were able to determine that the components in the dietary supplement have a positive effect in offsetting some of the factors that contribute to the early mortality of TGM. Projects to study pro-oxidant production, glial acidic fibrillary protein (in key areas of the brain), and insulin levels in supplemented TGM and their normal siblings are currently underway to further assess the effectiveness of the dietary supplement to ameliorate the targeted aging processes.
Acknowledgments
Address correspondence to Jennifer Lemon, Department of Biology, McMaster University, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1. E-mail: lemonja@mcmaster.ca
REFERENCES
Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61-75.
Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68:71-87.
Rollo CD, Carlson J, Sawada M. Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can J Zool. 1996;74:606-620.
Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT. Does growth hormone prevent or accelerate aging? Exp Gerontol. 1998;33:675-687.
Carlson JC, Bharadwaj R, Bartke A. Oxidative stress in hypopituitary dwarf mice and in transgenic mice overexpressing human and bovine GH. Age. 1999;22:181-186.
Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210-216.
Rollo CD. Growth negatively impacts the lifespan of mammals. Evol Dev. 2002;55:55-61.
Samaras TT, Elrick H. Height, body size and longevity. Acta Med Okayama. 1999;53:49-169.
Bartke A. Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size. Results Probl Cell Differ. 2000;29:181-202.
Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22-29.
Lemon JA, Boreham DR, Rollo CD.
A Dietary Supplement Abolishes Age-related Cognitive Decline in Transgenic Mice Expressing
Elevated Free Eadical Processes
Exp Biol Med (Maywood). 2003 (Jul); 228 (7): 800810Cartee GD, Bohn EE, Gibson BT, Farrar RP. Growth hormone supplementation increases skeletal muscle mass of old male Fischer 344/brown Norway rats. J Gerontol Biol Sci. 1996;51A:B214-B219.
Ariznavarreta C, Castillo C, Segovia G, Mora F, Azcoitia I, Tresguerres JA. Growth hormone and aging. Homo. 2003;54:132-141.
Meliska CJ, Burke PA, Bartke A, Jensen RA. Inhibitory avoidance and appetitive learning in aged normal mice: comparison with transgenic mice having elevated plasma growth hormone levels. Neurobiol Learn Mem. 1997;68:1-12.
Pendergrass WR, Li Y, Jiand D, Wolf NS. Decrease in cellular replicative potential in giant mice transfected with the bovine growth hormone gene correlates to shortened life span. J Cell Physiol. 1993;156:96-103.
Ogueta S, Olazabal I, Santos, I, Delgado-Baeza E, Garcia-Ruiz JP. Transgenic mice expressing bovine GH develop arthritic disorder and self-antibodies. J Endocrinol. 2001;165:321-328.
Hauck SJ, Bartke A. Free radical defenses in the liver and kidney of human growth hormone transgenic mice: possible mechanisms of early mortality. J Gerontol Biol Sci. 2001;56A:B153-B162.
Miller DB, Bartke A, O'Callaghan JP. Increased glial fibrillary acidic protein (GFAP) levels in the brains of transgenic mice overexpressing the bovine growth hormone (bGH) gene. Exp Gerontol. 1995;30:383-400.
Bollano E, Omerovic E, Bohlooly-y M, et al. Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology. 2000;141:2229-2235.
Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125-137.
Peten EP, Striker LJ, Fogo A, Ichikawa I, Patel A, Striker GE. The molecular basis of increased glomerulosclerosis after blockade of the renin angiotensin system in growth hormone transgenic mice. Mol Med. 1994;1:104-115.
Matsuo M, Gomi F, Dooley MM. Age-related alterations in antioxidant capacity and lipid peroxidation in brain, liver, and lung homogenates of normal and vitamin E-deficient rats. Mech Ageing Dev. 1992;64:273-292.
Goto S, Takahashi R, Araki S, Nakamoto H. Dietary restriction initiated in late adulthood can reverse age-related alterations of protein and protein metabolism. Ann N Y Acad Sci. 2002;959:50-56.
Parr T. Insulin exposure and aging theory. Gerontology. 1997;43:182-200.
Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574-3578.
Bartke A, Cecim M, Tang K, Steger AW, Chandrashekar V, Turyn D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. PSEBM 1994;206:345-357.
Dominici FP, Cifone D, Bartke A, Turyn D. Loss of sensitivity to insulin at early events of the insulin signalling pathway in the liver of growth hormone-transgenic mice. J Endocrinol. 1999;161:383-392.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298-300.
Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547-581.
Akiyama H, Barger S, Bradt B, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383-421.
Kajiura L, Rollo CD. A mass budget for transgenic supermice engineered with extra rat growth hormone genes: evidence for energetic limitation. Can J Zool. 1994;74:492-507.
Rollo CD, Lai M, Whitehead K, Perreault ML, Lemon J, Chaudhry AM. Thermoregulation of transgenic growth hormone mice. Can J Zoo. 2004;82:934-949.
Carney JM, Starke-Reed PE, Oliver CN, Landum AW, Cheng MS, Wu JF, et al. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-α-phenylnitrone. Proc Natl Acad Sci U S A. 1991;88:3633-3636.
Hagen TM, Ingersoll RT, Lykkesfeld J, et al. R-α-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13:411-418.
Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyle-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870-1875.
Atamna H, Robinson C, Ingersoll R, Elliott H, Ames BN. N-t-butyl hydroxylamine is an antioxidant that reverses age-related changes in mitochondria in vivo and in vitro. FASEB J. 2001;15:2196-2204.
Melov S. Therapeutics against mitochondrial oxidative stress in animal models of aging. Ann N Y Acad Sci. 2002;959:330-340.
Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356-2361.
Liu J, Atamna H, Kuratsune H, Ames BN. Delayed brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002;959:133-166.
Bohm F, Edge R, McGarvey DJ, Truscott TG. β-carotene with vitamins E and C offers synergistic cell protection against NOx. FEBS Lett. 1998;436:387-389.
Foley DJ, White LR. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3261-3263.
Olshansky SJ, Hayflick L, Carnes BA. No truth to the fountain of youth. Sci Amer. 2002;286:92-95.
Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor-I. Endocrinology. 1997;138:3515-3520.
Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001;25:311-323.
Rollo CD, Ko CV, Tyerman JGA, Kajiura L. The growth hormone axis and cognition: empirical results and integrated theory derived from giant transgenic mice. Can J Zoo. 1999;77:1874-1890.
Palmiter RD, Brinster RL, Hammer RE. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611-615.
Calder WA. Function and Life History. Cambridge, MA: Harvard University Press. 1999;431.
Jackson AU, Galecki AT, Burke DT, Miller RA. Mouse loci associated with life span exhibit sex-specific and epistatic effects. J Gerontol Med Sci. 2002;57A:B9-B15.

Return to NUTRITION
Since 10-29-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |