

A Complex Dietary Supplement Modulates Nitrative Stress
in Normal Mice and in a New Mouse Model of
Nitrative Stress and Cognitive AgingThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Mech Ageing Dev. 2012 (Aug); 133 (8): 523–529 ~ FULL TEXT
Jiangang Long, Vadim Aksenov. David Rollo, Jiankang Liu
Institute of Mitochondrial Biology and Medicine,
Department of Biology and Engineering,
The Key Laboratory of Biomedical Information Engineering of Ministry of Education,
Xi'an Jiaotong University School of Life Science and Technology,
Xi'an 710049, China.
We examined whether transgenic growth hormone mice (Tg) that exhibit accelerated cognitive aging and exceptional free radical damage also express elevated nitrative stress. We characterized age-related patterns of 3-nitrotyrosine (3-NT) in brain homogenate and mitochondria of Tg and normal (Nr) mice as modulated by a complex anti-aging dietary supplement. Levels of 3-NT rose rapidly with age in Tg brain homogenate whereas normal controls maintained constant lower levels. The age-related slope for 3-NT was 3.6-fold steeper in untreated Tg compared to treated Tg (p<0.009), although treated Tg showed elevation in youth. Opposite to Tg, treated Nr mice had reduced 3-NT in youth (p<0.02). The age-related pattern of mitochondrial 3-NT in Nr mice was parabolic (p<0.005). Remarkably, levels in treated Nr were reduced by ~50% (p<0.0007). Untreated Tg showed strongly increasing mitochondrial 3-NT with higher mitochondrial activity (p<0.01) whereas treated Tg showed lower nitrosylation at higher levels of mitochondrial activity. Tg mice also expressed a postural abnormality that is a biomarker of neurodegeneration and/or nitrative stress. Tg represent a promising new model of nitrative stress associated with brain deterioration and results provide proof of principle that complex dietary supplements may be ameliorating.
From the FULL TEXT Article:
Introduction
We assessed reactive nitrogen species damage in normal (Nr) and transgenic growth hormone mice (Tg) using 3-nitrotyrosine (3-NT). Elevated 3-NT is a reliable biomarker of nitrative stress, aging and associated pathologies (Radi, 2004), particularly cognitive and somatosensory dysregulation as occurs in Alzheimer’s, Parkinson’s and Huntington’s diseases (Ansari and Scheff, 2010; Butterfield et al., 2007; Calabrese et al., 2009; Knott and Bossy-Wetzel, 2009; Steinert et al., 2010). Peroxynitrite generated via interaction of superoxide and nitric oxide (NO) mainly contributes to 3-NT formation and also impacts membranes, catalytic activity, cytoskeletal organization, and cell signaling pathways (Nuss et al., 2009; Pacher et al., 2007).
Mitochondria are the primarily source of free radicals and substantially contribute to reactive nitrogen species (Wei, 1998). Mitochondrial neuronal nitric oxide synthase (mtNOS) generates NO in immediate proximity to high concentrations of superoxide, thus favoring formation of peroxynitrite (Finocchietto et al., 2009). A cytoplasmic nNOS may also contribute to reactive nitrogen species. While diffusion of NO is rapid and it can react with superoxide even in adjacent cells (Pacher et al., 2007), highest nitrative damage is likely to occur near the source. Therefore, we quantified mitochondrial- derived 3-NT separately from whole-cell homogenate.
Transgenic growth hormone mice (Tg) express elevated free radical processes in all tissues examined (Rollo et al., 1996) and display accelerated aging with pronounced declines in cognitive and motor functions (Aksenov et al., 2010; Lemon et al., 2003, 2005). We developed a complex dietary supplement (DSP) targeting five key mechanisms of aging (oxidative stress, inflammation, mitochondrial function, insulin resistance and membrane integrity). The DSP strongly ameliorated functional aging and age-associated pathologies in Tg and Nr mice (Aksenov et al., 2010; Lemon et al., 2003, 2005). Here we confirm Tg as a new model of nitrative stress and show that the DSP ameliorated nitrative processes in both genotypes (particularly in mitochondria). This suggests promise for neurodegenerative conditions where nitrative stress is one likely mechanism (Kanaan et al., 2008).
Materials and methods
Animals and diets

Table 1 Breeding and husbandry of random bred C57BL/6J*SJL Nr and Tg mice were previously described (Aksenov et al., 2010). Protocols adhered to Canada Council on Animal Care guidelines. Our DSP contained 30 ingredients (Table 1) available without prescription (Aksenov et al., 2010). Dosages were derived from recommended human doses adjusted for body size and the 10-fold higher metabolic rate of mice (Lemon et al., 2005). A thoroughly mixed slurry of the DSP was soaked onto small pieces of bagel. Mice from the breeding colony were randomly assigned at weaning and for life to either the DSP treatment group (one dose/day) or an untreated control group. Bagel bits were avidly eaten ensuring accurate dosing. Nr and Tg mice in appropriate age ranges were randomly selected from control and supplemented populations for study.
Preparation of brain homogenate and brain mitochondria
Brains were removed on ice and stored at –80°C. The tissues were homogenized in 0.05 M PBS with 1 mM 2-mercapitethanol, 1 mM EDTA and 0.1% Trion-x100 as described (Hinman and Blass, 1981). Brain mitochondria were prepared by Keeney’s method (Keeney et al., 2006), and protein concentration was determined with a bicinchoninic acid (BCA) kit.
Mitochondrial complex activities
Mitochondrial complex activities (I–IV) were determined as previously described (Long et al., 2009a,b). Numbers per group (male mice) were as follows: untreated Tg, 8; treated Tg, 11; untreated Nr, 10; and treated Nr, 11. Briefly, complex I activity was assayed by monitoring the decrease of NADH absorption at 340 nm. Final concentration of mitochondria protein was 30 µg/mL. Reaction was started by adding 200 µmol/L NADH and scanned at 340 nm for 3 min. Rotenone (3 µmol/L) was added into the reaction system as blank control. Complex II was assayed in the assay buffer (10x buffer contains 0.5 M phosphate buffer, pH 7.8, 1% BSA, 10 µM antimycin A, 2 mM NaN3, 0.5 mM coenzyme Q1) with mitochondria (final concentration 25 µg/mL). The reaction was started with 10 mM succinate and scanned at 600 nm for 2 min at 30°C. Complex III activity was measured in the mixture containing 250 mmol/L sucrose, 1 mmol/L EDTA, 50 mmol/L KPi, pH value adjusted to 6.5 to reduce auto-oxidation of reduced CoQ1, 2 mmol/L KCN, 50 µmol/ L cytochrome C, 0.1% BSA, and the reaction was initiated by 20 µg/mL brain mitochondria and 50 µmol/L reduced CoQ1, recording the increase of absorption at 550 nm for 2 min. Complex IV was measured by monitoring the decrease of reduced cytochrome C at 550 nm.
Slot-blot assays of 3-NT and 4-HNE in brain homogenates and mitochondria
3-NT and 4-HNE were measured by slot-blot (Opii et al., 2008; Sultana and Butterfield, 2008) and relative density was obtained via optical scans. Measurements were made for both brain homogenates and the mitochondrial fraction. The 3-NT and 4-HNE intensity were represented as relative density units (RDUs).
Tail hang and motor balance tests
For the tail-hang test mice were picked up by the base of the tail and slowly lowered towards the surface of a bench. Normal mice reach out their forelegs and splay their legs in anticipation of landing. Mice with neurodegenerative conditions like the Huntington mouse with high nitrative stress clasp their feet together and hold the legs close to the body instead of reaching. Motor function was assessed with the standard Rota-rod test. Mice were placed on a slowly (5 rpm) rotating cylindrical rod (6 cm in diameter). Latency to fall was recorded. A soft landing pad 40 cm below cushioned the fall. Each mouse was tested 4 consecutive times. The worst (shortest) performance was discarded and latencies of the remaining 3 trials were averaged to provide a biomarker of balance. A 2 min cutoff time was applied.
Statistical analyses
Global comparisons among all groups were performed using general linear models. Impacts of diet on Nr and Tg mice were also analyzed separately as otherwise different trends between genotypes (e.g., opposite patterns and nonlinear relationships) confounded resolution. Age-related trends were characterized with linear and polynomial regression. Analysis of covariance (ANCOVA) was applied to assess impacts of diet when covariates were significant (e.g. age and mitochondrial complex activity). In all cases separate slopes models were employed. Descriptive statistics included means ± standard error (SE). Analyses were performed with Statistica1 software.
Results
Homogenate 3-nitrotyrosine

Figure 1 Pooled Tg treatments showed an extremely steep age-related rise in homogenate 3-NT (r = 0.67, p < 0.002, Fig. 1A). This was particularly accentuated in untreated Tg that showed a regression slope 3.6-fold steeper than treated Tg (although untreated Tg also had a lower intercept and lower 3-NT in youth than treated Tg, Fig. 1A). Linear regression for untreated Tg was highly significant:
3-NT = –23:0872 + 16:6229 (Age), r = 0:84; p < 0:01:
The regression for treated Tg, however, was only marginally resolved (r = 0.58, p < 0.06) although overall significance was improved (p < 0.002) when Tg data were pooled. The DSP appeared to increase 3-NT in young Tg (Fig. 1A). ANCOVA for Tg mice found a highly significant main effect for the DSP (p < 0.01) and an even more significant DSP*Age interaction (p < 0.0005). This reflected the complexity of results (i.e., increases in youthful 3-NT for treated Tg, but possible amelioration in older ages) (Fig. 1A).
Homogenate 3-NT showed a weak but significant increase with age in pooled Nr:
Nr 3-NT = 2682:008 + 2:526 (Age), r = 0:45; p < 0:04:
The main effect of supplementation was not resolved (ANCOVA: p = 0.103) for Nr but a significant DSP*Age interaction emerged (p < 0.02). Untreated Nr showed stable age-related 3-NT (slope = 0.491, p > 0.75, Fig. 1B), but treated Nr mice showed significantly increasing 3-NT with age. Opposite to treated Tg, the DSP reduced 3-NT in younger Nr mice:
3-NT = 1883:95 + 5:038 (Age), r = 0:71; p < 0:01:
ANCOVA applied to all mice detected highly significant interaction effects for Genotype*DSP*Age (p < 0.00002) and Genotype*DSP (p < 0.001) for homogenate 3-NT. Although all Tg and Nr mice expressed similar levels of homogenate 3-NT in youth (<150 d), for mice older than 300 d, untreated Tg had 2-fold greater homogenate 3-NT than untreated Nr mice (p < 0.004, ttest). The slope for pooled Tg was 3.9-fold greater than pooled Nr mice and untreated Tg had a slope 6.6-fold steeper than pooled Nr mice. Overall mean 3-NT for transgenic mice (4951.27 ± 295.04) was 1.4-fold greater than for normal mice (3587.99 ± 277.43). Both pooled genotypes showed age-related increases in 3-NT and some impacts of supplementation. For untreated genotypes, however, Tg showed remarkably rising 3-NT whereas Nr mice maintained virtually constant levels of homogenate 3-NT into advanced ages. Overall, the DSP impacted Tg and Nr homogenate 3-NT oppositely. Moreover, all aspects were greatly accentuated in Tg mice.
Mitochondrial 3-nitrotyrosine
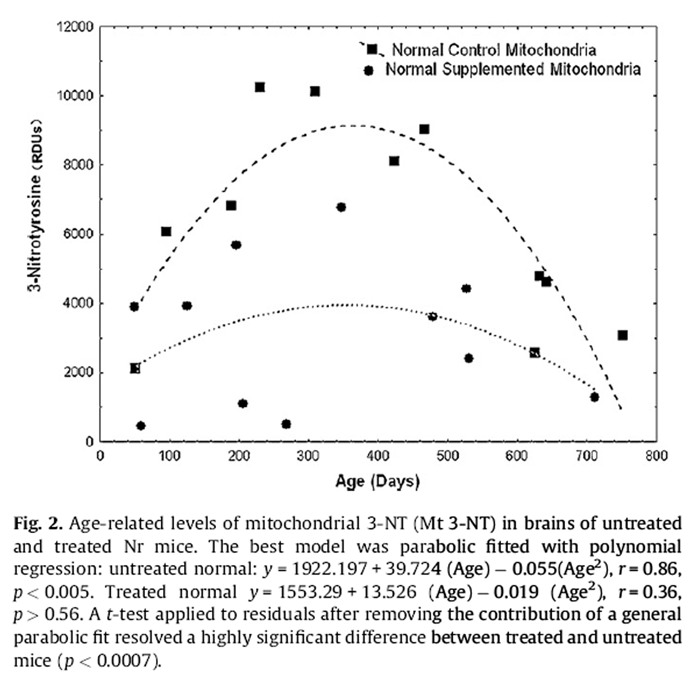
Figure 2 ANCOVA detected a significant genotype effect for mitochondrial 3-NT (p < 0.02) but no main effect of diet. Tg had 1.41-fold greater levels of mitochondrial 3-NT (6499.82 ± 605.14) than Nr mice (4616.50 ± 574.08). Pooled Tg data revealed a significant agerelated decline in mitochondrial 3-NT (p < 0.01, see dichotomy results below). An impact of diet on Tg mitochondrial 3-NT was marginally significant (ANCOVA, Age as covariate, p = 0.0509). Both treated and control Tg showed similar trends for age-related decline but a significant regression was only resolved for pooled data. Untreated Nr mice expressed a highly significant parabolic pattern of mitochondrial 3-NT with age (Fig. 2):
Mitochondrial 3-NT = 1922:187 + 39:724 (Age)
– 0:055 (Age2), r
= 0:86; p < 0:005:
A similar trend in treated Nr mice was not resolved. A simple comparison for dietary impacts on 3-NT in Nr mice without age as a covariate was statistically resolved (p < 0.01, t-test). Control Nr mice had mitochondrial 3-NT levels (6137.7 ± 2968.6) nearly double those of treated Nr mice (3095.3 ± 2121.7). We also compared the residuals for normal mitochondrial 3-NT after accounting for the general parabolic fit (first order polynomial) to the data (i.e., values obtained by subtracting the observed value from that predicted by the polynomial regression). This (equivalent to ANCOVA) resolved a highly significant impact of the supplement on Nr mitochondrial 3- NT (p < 0.0007).
Lipid peroxidation
Levels of mitochondrial lipid peroxidation (4-HNE) were positively associated with mitochondrial 3-NT in normal mice:
Mitochondrial 3-NT = 1419:61
+ 0:801 Imitochondrial 4-HNE), r
= 0:60; p < 0:003:
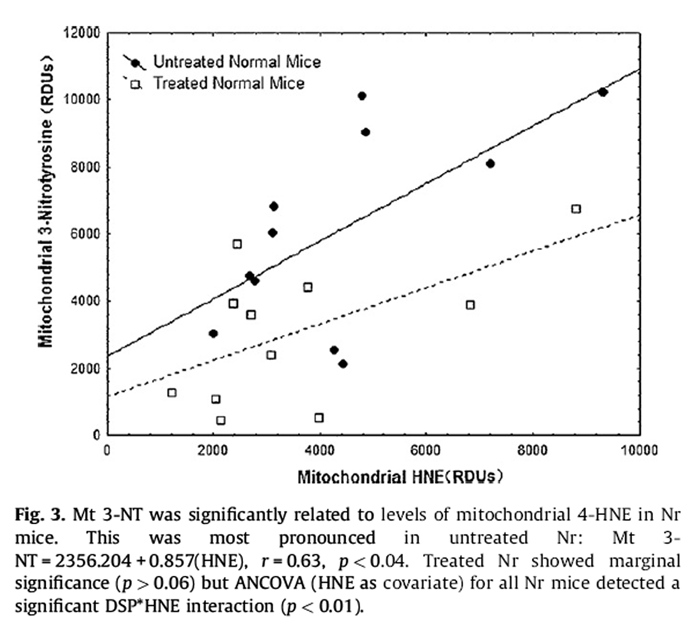
Figure 3 This was most pronounced in untreated Nr (Fig. 3):
Mitochondrial 3-NT = 2356:204 + 0:857(HNE), r
= 0:63; p < 0:04:
Treated Nr showed marginal significance (p > 0.06) but ANCOVA (HNE as covariate) for all Nr mice detected a significant DSP*HNE interaction (p < 0.01).
Tg showed a similar pattern that was not statistically resolved. ANOVA detected levels of homogenate 4-HNE in Tg 1.4-fold higher (4951.27 ± 418.52) than in Nr mice (3587.99 ± 393.99) (p < 0.02). ANCOVA did not resolve age as a covariate, but rising levels of 4-HNE were detected in normal homogenate:
Homogenate 4-HNE = 1772:92 + 1:291 (Age), r
= 0:50; p < 0:02:
Mitochondrial complex index
A general index of mitochondrial function (MI) was calculated by summing activity of complexes I–IV. Exploratory analyses found that the MI generally resolved clearer patterns and higher significance than individual complexes alone or an index calculated by multiplying across individual complexes.
Both treated and untreated Tg showed a trend for declining homogenate 3-NT with the MI. This was not statistically resolved using the MI index but pooled Tg were resolved using an index summing complexes III + IV:
Tg mouse homogenate 3-NT = 9257:66
– 256:199 (complex III + IV), r
= –0:52; p < 0:02
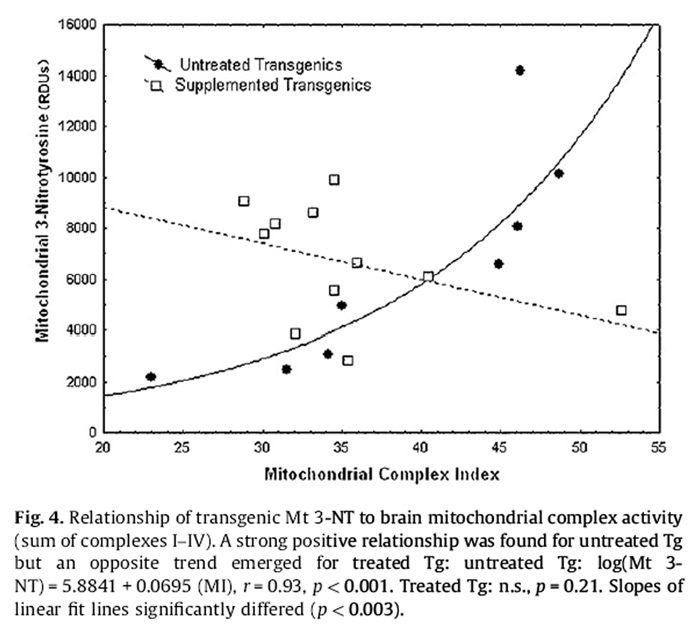
Figure 4 Untreated Nr 3-NT in homogenate showed a significant negative relationship with the MI, whereas treated Nr showed a non-significant trend for increase (Fig. 4):
Untreated Nr Homogenate 3-NT = 6505:0 – 57:387 (MI), r
= –0:67; p = 0:048:
Opposite to the homogenate, untreated Tg mitochondrial 3-NT showed a strong positive association with MI (Fig. 4):
Untreated Tg Mitochondrial 3-NT = –8459:38 + 385:23 (MI), r
= 0:84; p < 0:01:
The relationship appeared exponential, however, and a logarithmic transformation obtained a fit explaining 86% of the variance in mitochondrial 3-NT (i.e., r2 = 0.86):Untreated Tg (Log Mitochondrial 3-NT) = 5.8841 + 0.0695 (MI), r = 0.93, p < 0.001.
A slight negative trend in mitochondrial 3-NT was not resolved for treated Tg but ANCOVA with the MI as a covariate detected a strong positive main effect of the DSP (p < 0.002) and a DSP*MI interaction (p < 0.003). Complex III appeared to particularly contribute to this pattern but obtained lower statistical resolution (DSP: p < 0.02; DSP*Mitochondrial Complex III interaction: p < 0.03).
Nr mitochondrial 3-NT showed no clear relationship to the MI. However, for any level of MI, mitochondrial 3-NT was ~2-fold higher in untreated Nr mice (p < 0.01).
Homogenate versus mitochondrial 3-nitrotyrosine dichotomy
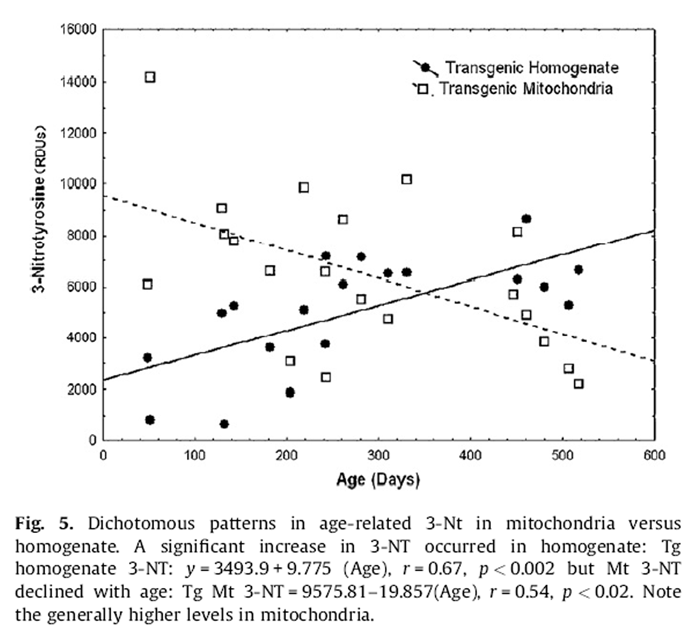
Figure 5 Tg expressed opposite age-related patterns of 3-NT in the mitochondrial compartment compared to homogenate. Homogenate levels increased by ~4-fold from youth to old age (Fig. 5):
Homogenate 3-NT = 2348:76 + 9:775 (Age), r
= 0:67; p < 0:002:
Alternatively, mitochondrial levels reflected a reciprocal (~5- fold) decline across the same age range (Fig. 5):
Mitochondrial 3-NT = 9575:81 – 10:857 (Age), r
= –0:54; p < 0:01:
For untreated Tg, mitochondrial 3-NT (6362.8 ± 1312.8) was 1.4-fold higher than in homogenate (4510.8 ± 1106.6). For untreated Nr mice 3-NT in mitochondria (6137.7 ± 895.1) was 1.7-fold higher than in homogenate (3689.9 ± 336.3). These patterns highlight the higher levels of 3-NT in mitochondria and that the greatest differences in 3-NT between untreated Nr and Tg are in homogenate. Remarkably, treated Nr mitochondrial 3-NT (3095.3 ± 639.7) was very similar to treated Nr homogenate 3-NT (3486.1 ± 466.7)(only 11% difference). For treated Tg mitochondrial 3-NT (6636.9 ± 683.4) was only 1.2-fold greater than 3-NT in homogenate (5391.7 ± 358.0). Thus, the supplement tended to equalize homogenate and mitochondrial 3-NT.
Behavioral biomarkers of neuropathology

Figure 6 The mouse model for Huntington’s disease (HD) has elevated nitrosylation in brain associated with a distinct postural abnormality common to some other neurodegenerative conditions (Tanaka et al., 2004). When hung by their tail Nr mice reach out their forelegs towards the surface and splay their hind legs (Fig. 6A). Huntington mice instead clasp their paws (which can include the hind feet) (Tanaka et al., 2004). Tg mice hung by their tails displayed identical paw clasping and leg tucking as found in the Huntington mouse (Fig. 6B).
In Huntington’s, accumulation of nitrosylated proteins in the cerebellum results in altered motor function and balance. A standard test for assessing motor balance involves placing a mouse on a slowly rotating cylindrical rod (rotarod) (Hamm et al., 1994). At 5 rpm, this is a mild challenge for Nr mice. Untreated Nr successfully remained on the rotarod for up to 2 min but Tg fell within ~30 s (~75% sooner than Nr controls (t-test: p < 0.00001, Fig. 6C).
diseases. This may be particularly true for HD. Whereas Nr mice engage a reaching response when hung by their tail (Fig. 6A). HD mice express the same paw clasping behavior in the tail hang test as do Tg (Fig. 6B) (Komatsu et al., 2006). HD mice are a model of nitrative stress and dysregulated motor balance (Tanaka et al., 2004). Tg similarly performed very poorly on the rotarod (Fig. 6C). Although this is considered a standard assay, caution is required as the exceptional size of Tg may also influence their coordination on this task and Tg expressed no other obvious symptoms of HD.
Discussion
Mitochondrial complex index
The index, summing activity of complexes I–IV, is believed better representing the activity of mitochondrial electron transport chain than the individual complex activity. It resolved clearer patterns and higher significance than individual complexes alone in present study. Mitochondrial 3-NT (Mt 3-NT) increased exponentially with increasing MI in untreated Tg (Fig. 4). Supplementation abolished the strong coupling of 3-NT production to the MI, although it resulted in higher levels of 3-NT associated with low mitochondrial activity (Fig. 4). ANCOVA detected significant DSP (p < 0.002) and DSP*MI effects (p < 0.003). Complex III appeared to particularly contribute to this pattern as we previously reported for protein carbonyls (Aksenov et al., 2010).
Nr Mt 3-NT showed no relationship to the MI in terms of slope. However, for any level of MI, mitochondrial 3-NT was ~2-fold higher in untreated Nr mice (i.e., parallel flat lines differed in intercepts). The fact that MI explains 86% of the variance in mitochondrial 3-NT in untreated Tg, whereas Nr mice show no agerelated pattern suggests that a regulatory mechanism acting in Nr mice is dysregulated in Tg.
Homogenate versus mitochondrial 3-nitrotyrosine dichotomy
Complex and sometimes opposite relationships of 3-NT and the MI also emerged for age-related patterns of homogenate versus mitochondrial 3-NT. Because mitochondria are the major source of superoxide, nitrative stress might be expected to trace to mitochondria. Mitochondria indeed expressed higher levels of 3-NT than homogenate in both Nr and Tg. Regardless, we found remarkably opposite age-related patterns of 3-NT in Tg mitochondria (linear decline) compared to homogenate (linear increase) (Fig. 5). We previously found that patterns of protein carbonyls (a marker of oxidative stress) also showed different patterns in Tg homogenate (U-shaped) versus mitochondria (a trend for age-related decline) (Aksenov et al., 2010).
One possibility is that homogenate 3-NT mainly reflects membrane-bound NAD(P)H oxidases and cytosolic nitric oxide synthase. (Komatsu et al., 2006; Lemon et al., 2005; Rollo, 2007). A further possibility is that mitochondrial turnover and/or declining mitochondrial activity underlie the mitochondrial pattern, whereas failure to remove damaged proteins (perhaps exacerbated by ATP shortfalls) results in accumulating damage in the homogenate. In the latter case, the association of the DSP with reduced dichotomy in homogenate versus mitochondrial 3-NT (particularly in Nr mice) could well reflect a benefit for the proteosome. Differences in patterns of 3-NT compared to previous results for protein carbonyls also suggest that various reactive oxygen species may differ in their impacts with age. Regardless, maximal homogenate carbonylation and nitosative stress converge in senescent ages in Tg.
Lipid peroxidation
Levels of mitochondrial lipid peroxidation (4-HNE) were positively associated with Mt 3-NT in normal mice (p < 0.003). This was more pronounced in untreated Nr and ANCOVA (HNE as covariate) detected a significant DSP*HNE interaction (p < 0.01, Fig. 3). This could mean that the DSP partially acts on Mt 3-NT by reducing levels of mitochondrial damage. Tg showed a similar positive trend for Mt 3-NT with 4-HNE that was not statistically resolved. Although the free radical and mitochondrial theories of aging are under strong scrutiny, these results suggest that more free radicals may be generated by damaged mitochondria. In homogenate, Tg had 1.4-fold higher levels of 4-HNE than Nr mice (p < 0.02) and rising levels with age were similar in treated and untreated Nr mice and resolved when Nr were pooled (p < 0.02).
Behavioral biomarkers of neuropathology
Tg are a model of accelerated cognitive aging (Aksenov et al., 2010; Rollo et al., 1996) of likely relevance to Alzheimer’s, Parkinson’s and Huntington’s (HD)
Conclusion
Collectively, our results provide a new model linking nitrative stress to cognitive dysfunction and we demonstrate the utility of Tg by exploring the potential of dietary supplements to modulate reactive nitrogen processes in brain. The elevation of homogenate 3-NT in untreated Tg is profound whereas untreated Nr maintain stable levels during aging. Our analyses demonstrate that various aging biomarkers can express surprisingly complex non-linear temporal patterns and spatial compartmentalization requiring careful statistical dissection. Resolution requires samples spanning the lifetime of groups. Tg are likely to prove valuable for understanding neurodegenerative conditions associated with free radical and nitrative stress (e.g., Alzheimer’s, Parkinson’s and Huntington’s). Peroxynitrite is also implicated in many other pathological processes including diabetes where amelioration improves cardiac and vascular pathology (Obrosova et al., 2005). Elevations in the GH axis are recognized as promoting aging (Brown-Borg, 2009) and our results further confirm the transgenic growth hormone mouse as a model of accelerated aging (Aksenov et al., 2010; Lemon et al., 2003, 2005; Rollo et al., 1996).
Acknowledgments
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to CDR, and from the National Natural Science Foundation of China to JGL (Grant No. 31070740). We also thank Dr. Brian McCarry (Chair of Biology, McMaster University) for financial support for graduate students.
Disclosure statement: No actual or potential conflicts of interest.
REFERENCES
Aksenov, V., Long, J., Lokuge, S., Foster, J.A., Liu, J., Rollo, C.D., 2010.
Dietary amelioration of locomotor, neurotransmitter and mitochondrial aging.
Experimental Biology and Medicine (Maywood) 235, 66–76Ansari, M.A., Scheff, S.W., 2010.
Oxidative stress in the progression of Alzheimer disease in the frontal cortex.
Journal of Neuropathology and Experimental Neurology 69, 155–167Brown-Borg, H.M., 2009.
Hormonal control of aging in rodents: the somatotropic axis.
Molecular and Cellular Endocrinology 299, 64–71Butterfield, D.A., Reed, T.T., Perluigi, M., De Marco, C., Coccia, R., Keller, J.N., Markesbery, W.R., Sultana, R., 2007.
Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease.
Brain Research 1148, 243–248Calabrese, V., Cornelius, C., Rizzarelli, E., Owen, J.B., Dinkova-Kostova, A.T., Butterfield, D.A., 2009.
Nitric oxide in cell survival: a janus molecule.
Antioxidants and Redox Signaling 11, 2717–2739Finocchietto, P.V., Franco, M.C., Holod, S., Gonzalez, A.S., Converso, D.P., Antico Arciuch, V.G., Serra, M.P., Poderoso, J.J., Carreras, M.C., 2009.
Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death.
Experimental Biology and Medicine (Maywood) 234, 1020–1028Hamm, R.J., Pike, B.R., O’Dell, D.M., Lyeth, B.G., Jenkins, L.W., 1994.
The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury.
Journal of Neurotrauma 11, 187–196Hinman, L.M., Blass, J.P., 1981.
An NADH-linked spectrophotometric assay for pyruvate dehydrogenase complex in crude tissue homogenates.
Journal of Biological Chemistry 256, 6583–6586Kanaan, N.M., Kordower, J.H., Collier, T.J., 2008.
Age-related changes in dopamine transporters and accumulation of 3-nitrotyrosine in rhesus monkey midbrain dopamine neurons: relevance in selective neuronal vulnerability to degeneration.
European Journal of Neuroscience 27, 3205–3215Keeney, P.M., Xie, J., Capaldi, R.A., Bennett Jr., J.P., 2006.
Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled.
Journal of Neuroscience 26, 5256–5264Knott, A.B., Bossy-Wetzel, E., 2009.
Nitric oxide in health and disease of the nervous system.
Antioxidants and Redox Signalling 11, 541–554Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., Ueno, T., Koike, M., Uchiyama, Y., Kominami, E., Tanaka, K., 2006.
Loss of autophagy in the central nervous system causes neurodegeneration in mice.
Nature 441, 880–884Lemon, J.A., Boreham, D.R., Rollo, C.D., 2003.
A Dietary Supplement Abolishes Age-related Cognitive Decline in Transgenic Mice Expressing
Elevated Free Eadical Processes
Exp Biol Med (Maywood). 2003 (Jul); 228 (7): 800–810Lemon, J.A., Boreham, D.R., Rollo, C.D., 2005.
A Complex Dietary Supplement Extends Longevity of Mice
J Gerontol A Biol Sci Med Sci. 2005 (Mar); 60 (3): 275–279Long, J., Gao, F., Tong, L., Cotman, C.W., Ames, B.N., Liu, J., 2009a.
Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine.
Neurochemical Research 34, 755–763Long, J., Gao, H., Sun, L., Liu, J., Zhao-Wilson, X., 2009b.
Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson’s disease model.
Rejuvenation Research 12, 321–331Nuss, J.E., Amaning, J.K., Bailey, C.E., DeFord, J.H., Dimayuga, V.L., Rabek, J.P., Papaconstantinou, J., 2009.
Oxidative modification and aggregation of creatine kinase from aged mouse skeletal muscle.
Aging 1, 557–572Obrosova, I.G., Mabley, J.G., Zsengeller, Z., Charniauskaya, T., Abatan, O.I., Groves, J.T., Szabo, C., 2005.
Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst.
FASEB Journal 19, 401–403Opii, W.O., Joshi, G., Head, E., Milgram, N.W., Muggenburg, B.A., Klein, J.B., Pierce, W.M., Cotman, C.W., Butterfield, D.A., 2008.
Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease.
Neurobiology of Aging 29, 51–70Pacher, P., Beckman, J.S., Liaudet, L., 2007.
Nitric oxide and peroxynitrite in health and disease.
Physiological Reviews 87, 315–424Radi, R., 2004.
Nitric oxide, oxidants, and protein tyrosine nitration.
Proceedings of the National Academy of Sciences of the United States of America 101, 4003–4008Rollo, C., 2007.
Overview of research on giant transgenic mice with emphasis on the brain and aging.
In: Samaras, T. (Ed.), Human Body Size and the Laws of Scaling.
Nova Science Publishers, New York, pp. 235–260Rollo, C.D., Carlson, J., Sawada, M., 1996.
Accelerated aging of giant transgenic mice is associated with elevated free radical processes.
Canadian Journal of Zoology 74, 606–620Steinert, J.R., Chernova, T., Forsythe, I.D., 2010.
Nitric oxide signaling in brain function, dysfunction, and dementia.
Neuroscientist 16, 435–452Sultana, R., Butterfield, D.A., 2008.
Slot-blot analysis of 3-nitrotyrosine-modified brain proteins.
Methods in Enzymology 440, 309–316Tanaka, M., Machida, Y., Niu, S., Ikeda, T., Jana, N.R., Doi, H., Kurosawa, M., Nekooki, M., Nukina, N., 2004.
Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease.
Nature Medicine 10, 148–154Wei, Y.H., 1998.
Oxidative stress and mitochondrial DNA mutations in human aging.
Proceedings of the Society for Experimental Biology and Medicine 217, 53–63.
Return to NUTRITION
Since 8–08-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |