

Reporting of Adverse Events Associated with Spinal
Manipulation in Randomised Clinical Trials:
An Updated Systematic ReviewThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2023 (May 4); 13 (5): e067526 ~ FULL TEXT
OPEN ACCESS Lindsay M Gorrell, Benjamin T Brown, Roger Engel, and Reidar P Lystad
Integrative Spinal Research Group,
Department of Chiropractic Medicine,
University Hospital Balgrist and
University of Zurich,
Zurich, Switzerland
Objectives: To describe if there has been a change in the reporting of adverse events associated with spinal manipulation in randomised clinical trials (RCTs) since 2016.
Design: A systematic literature review.
Data sources: Databases were searched from March 2016 to May 2022: MEDLINE (Ovid), Embase, CINAHL, ICL, PEDro and Cochrane Library. The following search terms and their derivatives were adapted for each platform: spinal manipulation; chiropractic; osteopathy; physiotherapy; naprapathy; medical manipulation and clinical trial.
Methods: Domains of interest (pertaining to adverse events) included: completeness and location of reporting; nomenclature and description; spinal location and practitioner delivering manipulation; methodological quality of the studies and details of the publishing journal. Frequencies and proportions of studies reporting on each of these domains were calculated. Univariable and multivariable logistic regression models were fitted to examine the effect of potential predictors on the likelihood of studies reporting on adverse events.
Results: There were 5399 records identified by the electronic searches, of which 154 (2.9%) were included in the analysis. Of these, 94 (61.0%) reported on adverse events with only 23.4% providing an explicit description of what constituted an adverse event. Reporting of adverse events in the abstract has increased (n = 29, 30.9%) while reporting in the results section has decreased (n = 83, 88.3%) over the past 6 years. Spinal manipulation was delivered to 7518 participants in the included studies. No serious adverse events were reported in any of these studies.
Conclusions: While the current level of reporting of adverse events associated with spinal manipulation in RCTs has increased since our 2016 publication [26] on the same topic, the level remains low and inconsistent with established standards. As such, it is imperative for authors, journal editors and administrators of clinical trial registries to ensure there is more balanced reporting of both benefits and harms in RCTs involving spinal manipulation.
Keywords: adverse events; back pain; clinical trials; musculoskeletal disorders; rehabilitation medicine; spine.
Strengths And Limitations Of This Study
This systematic review is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
The search strategy was inclusive of professions that deliver spinal manipulation.
The search included several databases relevant to manual therapy.
Due to heterogeneity of reporting of adverse events, only descriptive statistics were used to describe domains of interest.
From the FULL TEXT Article:
Introduction
The use of high-velocity, low-amplitude (HVLA) spinal manipulation to treat spinal pain and dysfunction is recommended in clinical and best practice guidelines [1–4] and is commonly used by several healthcare professions. [5–7] Despite this, concerns remain surrounding adverse events following the intervention. [8, 9] Adverse events associated with spinal manipulation are typically benign, transient and do not require further treatment. [10] Indeed, some authors classify increased muscle soreness or stiffness in the treatment area as an ‘expected outcome of treatment’ rather than an adverse event. [11] At the other end of the spectrum, catastrophic events, such as vertebral artery dissection, have been temporally associated with spinal manipulation. [12] However, such events are rare, and as a result, are typically reported in individual case reports or case series with little to no information regarding the intervention that was delivered. [13] Indeed, synthesis of the current literature suggests that there is no evidence for cervical spine manipulation causing cervical artery dissection. [14] Additionally, several large population-based studies have reported that there is no difference in risk of cervical artery dissection following visits to a chiropractor compared with those occurring following a visit to a primary care provider [15, 16] or, in those who received cervical spinal manipulation compared with matched controls. [17, 18] Furthermore, recent biomechanical studies report that head angular displacements and vertebral artery length changes are small during cervical spine manipulation thrusts [19] and that the vertebral artery does not experience longitudinal force during cervical spine manipulation. [20] Despite this literature, the serious nature of such events that are temporally associated with cervical spine manipulation makes it imperative that the circumstances surrounding such events are reported transparently.
Randomised clinical trials (RCTs) are the gold standard study design for measuring effectiveness (benefit/s) of interventions for the treatment of spinal pain and dysfunction. However, as the risks of an intervention are also important to both patients and practitioners, RCTs should report on not only the efficacy of spinal manipulation, but also any adverse events associated with the intervention. The Consolidated Standards of Reporting Trials (CONSORT) statement, first published in 1996 with several updates since, provides the scientific community (specifically researchers and journal editors) with a scaffold to standardise and improve the quality of RCT reporting. [21–23] The CONSORT statement acknowledges the importance of reporting adverse events alongside effectiveness data. The 2004 Harms extension document [24] provides specific recommendations for how and where these data should be included in scientific manuscripts. However, reporting of adverse events in RCTs in the wider medical literature remains insufficient since the publication of the 2004 extension, [25] a finding that is also evident in RCTs that involve spinal manipulation. [26] Thus, the objective of this review was to describe if there has been a change in the reporting of adverse events associated with spinal manipulation in RCTs since 2016.
Methodology
This systematic literature review is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. [27]
Definitions
Spinal manipulation was defined as a manual procedure involving an HVLA thrust delivered to a spinal joint with the intention of moving the joint past its physiological range of motion but without exceeding the anatomic limit. [28] For the purposes of this review, spinal manipulation delivered using drop-piece-table and mechanical implements (eg, Activator instrument) were considered HVLA procedures. [29]
An adverse event was defined as any unfavourable reaction with a temporal association to spinal manipulation that resulted in an alteration in a participant’s activities of daily living, [30, 31] irrespective of the timing of onset, duration or severity of the event. [32]
A serious adverse event was defined as any unfavourable sign, symptom or disease temporally associated with the treatment, whether or not caused by the treatment that results in death or is life threatening or results in inpatient hospitalisation or prolongation of existing hospitalisation for more than 24 hours with a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions. [30]
To be classified as reporting on adverse events ‘directly’, a study must have provided explicit description of their operational definition of an adverse event (eg, ‘In the current study, an adverse event was defined as a sequelae of 1–week duration with any symptom perceived as distressing and unacceptable to the patient that required further treatment [excerpt from reference 63].’ [33]), and/or how data on adverse events were measured (eg, ‘Active and passive surveillance methods were used to collect information on adverse events.’ [34]), and/or provide a substantial description of adverse events observed during data collection. [35, 36] In contrast, all other studies reporting on adverse events ‘indirectly’ did not explicitly provide such information.
Patient and public involvement
No patients were involved in this systematic literature review.
Eligibility criteria
Consistent with the 2016 review, [26] RCTs reporting original data on spinal manipulation as either the sole intervention, or as the sole intervention in a comparator group, delivered by any regulated health professional, and published in English, were eligible for inclusion. Studies reporting on reviews, other trial designs, trial registrations, protocols, commentaries, editorials and conference proceedings were excluded. Further exclusion criteria included retracted articles, secondary analyses, studies in which the full text was not available in English and studies where manipulation was only applied to an area other than the spine. Studies were also excluded if it was unclear if the intervention being delivered involved an HVLA manipulation.
Search strategy
The following databases were searched from 1 March 2016 to 12 May 2022: MEDLINE (Ovid), Embase, CINAHL, ICL, PEDro and Cochrane Library. Reference lists of included studies were screened to insure all relevant literature was captured. The following search terms and derivatives were adapted for each platform: spinal manipulation; chiropractic; osteopathy; physiotherapy; naprapathy; medical manipulation and clinical trial. An example of each search strategy is provided in Online Appendix 1.
Study selection process
Records retrieved from the electronic searches were exported to the Rayyan online platform. [37] Duplicate records, and records included in the 2016 review, were removed before title and abstract screening. Two authors (LMG and BTB) independently screened included studies in a stepwise process, beginning with review of each title and abstract. Full texts of the studies remaining after this step were retrieved and further screened against the eligibility criteria (LMG and RE). Any disagreements regarding inclusion were resolved by consensus and if consensus could not be reached, disagreements were resolved by a third author (BTB).
Data extraction
Adverse events reporting data were extracted from the remaining studies by two authors (LMG and RPL). These data included descriptive information (ie, title, author, year of publication, country where the data was collected, journal of publication, spinal region treated (eg, cervical spine) and type of practitioner delivering the spinal manipulation (eg, chiropractor)), whether the study reported on adverse events (ie, reported/not and if reported, directly/indirectly), location of reporting within the article, classification of adverse events reported (eg, mild, moderate, serious and severe), completeness of adverse events reporting (ie, onset, duration and number of events reported), number of participants in the spinal manipulation group/s and descriptions of any definitions and/or classification systems used. Other data collated by the lead author (LMG) included whether the study was published in a journal that follows the International Committee of Medical Journal Editors (ICMJE) guidelines via a search of the ICMJE website [38] on 29 May 2022. Additionally, the most recently published impact factor (year 2020) for each journal was manually extracted by the lead author (LMG) from the Clarivate Journal Citations Reports website [39] on 29 May 2022.
Assessment of risk of bias using the Cochrane ROB V.2 assessment tool [40] was performed by three authors working in pairs (LMG and RE, and LMG and BTB) for all included studies to assess the methodological quality of the publication. Disagreements were resolved by consensus and if consensus could not be reached, disagreements were resolved by a third author (RPL).
Data analysis
Data were analysed using descriptive statistics. Frequencies and proportions of studies reporting on each of the specified domains above were calculated in Microsoft Excel (V.2102). Continuous variables with highly skewed distributions (ie, journal impact factor and sample size of spinal manipulation group) were categorised into tertiles. Univariable and multivariable logistic regression models were fitted to examine the effect of potential predictors on the likelihood of studies reporting on adverse events. The multivariable logistic regression model was fitted using backward elimination, whereby the least significant potential predictors were sequentially eliminated from the multivariable model until only significant predictors remained. The observed effects from the univariable and multivariable logistic regression models were reported as ORs and adjusted ORs (aORs), respectively, with 95% CIs. All statistical analyses were performed using the statistical computing software R V.4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
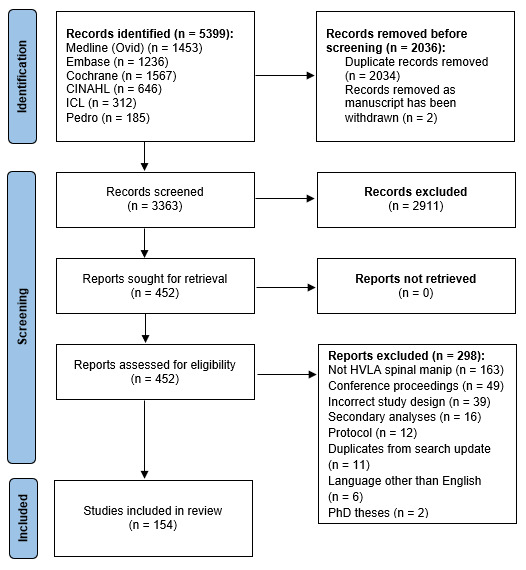
Figure 1 There were 5,399 records initially identified by the electronic searches (Figure 1). A total of 3,363 unique records remained after de-duplication (n = 2,034) and the removal of retracted articles (n = 2). After title and abstract screening, full texts of the 452 remaining studies were screened. Of these, 154 fulfilled the eligibility criteria and were included in the analysis (see Online Appendix 2 ). The most common reasons for exclusion were: the intervention did not consist of HVLA spinal manipulation (n = 163) and/or the study related to a conference proceeding (n = 49).
Comprehensiveness of reporting of adverse events

Table 1 Of the 154 included studies, 94 (61.0%) reported on adverse events. Of these 94 studies, 36 (38.3%) directly reported on adverse events, with studies in which spinal manipulation was delivered by a chiropractor most frequently reporting these data (n = 17; 47.2%, Table 1). Indirect reporting occurred in 58 studies (61.7%), with studies in which spinal manipulation was delivered by a physiotherapist being the most frequent (n = 29; 50.0%, table 1). Of the 60 studies (39.0%) that did not report on adverse events, studies in which spinal manipulation was delivered by a physiotherapist were the most frequent (n = 28; 46.7%, table 1). A description of what constituted an adverse event definition and/or the classification system used was provided in 22 studies (23.4%). However, most studies did not provide a description and instead used terms such as ‘adverse event’ (n = 70, 74.5%), ‘adverse effect’ (n = 22, 23.4%), ‘’side effect’ (n = 19, 20.2%) and ‘harm’ (n = 11, 11.7%) without adequate explanation. When mentioned, terms pertaining to classification systems (predominantly severity) were (number of studies in which the term was used, %): ‘mild’ (n = 20, 21.3%), ‘moderate’ (n = 17, 18.1%), ‘serious’ (n = 27, 28.7%) and ‘severe’ (n = 14, 14.9%). The onset of an adverse event/s was unclear in 30 (31.9%) studies. Duration of adverse events were reported heterogeneously, with some studies providing a time from either baseline or the start of intervention, whereas others provided a temporal descriptor such as ‘short-term’, ‘temporary’ or ‘transient’. Of the 9 studies providing times, durations were as follows: <72 hours (n = 3, 3.2%), >72 hours (n = 2, 2.1%) or mixed duration (n = 4, 4.3%). An evaluation tool was mentioned in 26 (27.7%) studies.
Number and location of adverse events reporting
No serious adverse events were reported in any of the 154 included studies, representing 7,518 participants who received spinal manipulation. Furthermore, of the 94 studies reporting on adverse events, 63 (67.0%) reported that no adverse events occurred. Adverse events were reported in the abstract of 29 (30.9%) and results section of 83 (88.3%) studies. Furthermore, adverse events were mentioned in several locations throughout the included studies: the introduction (n = 15, 16.0%), methods (n = 56, 59.6%), discussion (n = 30, 31.9%), conclusion (n = 7, 7.4%) and supplementary materials (n = 1, 1.1%).
Descriptors of studies reporting on adverse events

Table 2 Descriptive statistics are provided in Table 2. Of the 94 studies reporting on adverse events, 55 (58.5%) were rated at a ‘high risk of bias’, 29 (30.9%) as ‘some concerns’ and 10 (10.6%) at a ‘low risk of bias’ (online supplemental appendix 3). Additionally, 33 (35.1%) were published in journals stating that they follow the ICMJE recommendations. For the remaining studies, the median of the most recently published (2020) impact factor was 2.5 (IQR: 2.1–4.2).
Predictors for the reporting of adverse events

Table 3 There was very strong evidence that studies with an impact factor in the upper (aOR: 5.72 (95% CI 2.23 to 15.85); p<0.001) and middle (aOR: 3.52 (95% CI 1.51 to 8.57); p=0.004) tertiles were more likely to report on adverse events than those in the lower tertile when the model was adjusted for risk of bias, impact factor, spinal region of manipulation and number of participants receiving spinal manipulation (Table 3). There was also strong evidence that studies in which a chiropractor delivered the spinal manipulation were more likely to report on adverse events (aOR: 4.58 (95% CI 1.14 to 20.24); p=0.036). Studies in which spinal manipulation was delivered to more than one region or, it was unclear which regions the manipulations were delivered, were also more likely to report on adverse events (aOR: 3.18 (95% CI 1.16 to 9.05); p=0.027). While not achieving statistical significance, another factor of note included studies in which cervical spine manipulation was delivered (aOR: 3.04 (95% CI 0.88 to 11.30); p=0.085).
Discussion
There has been a change in the reporting of adverse events associated with spinal manipulation in RCTs since 2016. Specifically, the percentage of included studies reporting adverse events has increased from 38.0% (2016 study [26]) to 61.0% (current study). However, the current review highlights that the reporting of adverse events in RCTs involving spinal manipulation as an intervention remains poor and is not consistent with established standards. Specifically, of the 154 included studies, just over half (n = 94, 61.0%) reported on adverse events. Furthermore, of these 94 studies, less than half (38.3%) reported directly on adverse events, with only 23.4% providing an explicit description of what constituted an adverse event. Further complicating this issue is the vast heterogeneity of terms (ie, ‘adverse effect’, ‘side effect’, ‘harm’, etc) used to describe adverse events. This is disappointing given that there have been many calls in the literature for the improvement of adverse events reporting in RCTs, and for the development and use of standardised definitions and classification systems. [24, 26, 32, 41–46]
A recent scoping review explores the complexity of the current literature reporting on adverse events associated with spinal and peripheral joint manipulation and mobilisation. [47] Specifically, the authors report that conflicting opinions regarding facets of adverse event definition and classification such as: symptom severity and duration, relatedness to the intervention (eg, time to onset and treatment provided), action taken to treat the symptoms and expectedness, which profession delivered the intervention and geographical location (with possible medico-legal constraints and/or different expectations of reporting/not reporting), are all factors to reflect on when considering adverse events associated with joint manipulation and mobilisation. In an attempt to address the lack of standardised definitions and classification systems across professions that deliver spinal manipulation, the same authors have conducted an international Delphi study (manuscript in preparation; protocol paper [41]) to determine, by expert consensus, a standardised definition and severity classification for adverse events associated with spinal and peripheral joint manipulation and mobilisation. The development and use of such guidelines would constitute an important step toward uniform reporting of adverse events associated with spinal manipulation across all stakeholder professions and geographical locations.
However, until this work is published, online supplemental appendix 2 of the 2004 CONSORT Harms extension [24] provides a checklist of items to include and specific examples of good reporting when reporting on harms (adverse events) in RCTs. Furthermore, it appears that an update to this guideline is emergent. [25] It is hoped that these updated guidelines will ensure that authors and journal editors alike are both aware of and implement better harms reporting in the future. We strongly encourage researchers and journal editors alike to read and use the most recent CONSORT Harms checklist during all phases of study development, data collection, manuscript preparation, submission and during the review process. One important item on this checklist is that both benefits and harms should be stated in either the title and/or abstract of a manuscript. This point is salient as the abstract is the second-most read section of a scientific manuscript after the title. [48] Encouragingly, the reporting of adverse events in the abstract has doubled (15.7%–30.9%, 2016–current) when compared with our previous review of the literature. [26] Despite this, the current reporting on adverse events in the title/abstract of RCTs using spinal manipulation remains poor, a finding that is also present in the wider published medical literature discussing adverse events. [49–52] Despite an overall increase in the number of studies reporting on adverse events in RCTs involving spinal manipulation (38.0%–61.0%, 2016 [26]–current), adverse events reporting in the results section has decreased (93.6% vs 88.3%) over the past 6 years and remains lower than that in the wider published literature. [50, 53] It is unknown why there would be a decrease in the reporting on adverse events associated with spinal manipulation in one section of a scientific manuscript that it could reasonably be expected to be reported. Furthermore, an important source of information for the formulation of a considered evidence-based risk-benefit analysis for the use of spinal manipulation as a treatment option by both clinician and patient [49, 52] is transparent data reporting on both the efficacy and adverse events occurring in RCTs involving spinal manipulation.
Consistent with the literature, [31, 32, 42, 43, 47] there was considerable heterogeneity of nomenclature used to describe adverse events associated with spinal manipulation. Similar terms were used to indicate an adverse event in the current (compared with 2016) review: ‘adverse event’ (2016—73.0%; 2022—74.5% of studies), ‘adverse effect’ (23.6%; 23.4%), ‘side effect’ (21.3%; 20.2%) and ‘harm’ (16.4%; 11.7%). Additionally, while similar terms were used to describe classification systems previously reported (ie, ‘serious’, ‘mild’, ‘moderate’ and ‘severe’), these terms were rarely defined, which is consistent with the existing literature. [26, 52] Additionally, when present, the reporting of onset and duration of adverse events was inconsistent, again highlighting that there is an urgent need for the development of a standardised definition and classification system for the reporting of adverse events. [41] Furthermore, the responsibility for improved reporting of adverse events falls not only to authors but also to custodians of clinical trial registries and journal editors to ensure that there are provisions in study protocols for the adequate capture of adverse events and also that these events are adequately reported. that is, using the most recent CONSORT Harms extension guidelines, [24] alongside efficacy/effectiveness data. [25, 46, 54]
Manuscript reviewers and journal editors must be aware of the current best practices for the reporting of harms [24] and enforce these guidelines during peer review processes of both protocol and end-of-study results papers. However, this may not be as straight-forward as it appears. Despite this, there is a need for improved reporting of adverse events in RCTs that include spinal manipulation as an intervention and a first step would be for journals to incorporate clear instructions on harms reporting in their guidelines and instructions to authors. As a second step, journal editors may facilitate this process by limiting publication to only those studies that adhere to the current guidelines for the reporting of harms in RCTs that include spinal manipulation as an intervention. Indeed, if this was to occur, authors would need to ‘step-up’, to use expanded methodologies, reporting and statistical analyses that allow for the capture and reporting of adverse events data in RCTs that include spinal manipulation as an intervention. Specifically, data on adverse events should be actively collected as it has been reported that passive surveillance leads to an under-reporting [25, 54] and appropriate statistical analysis plans should be used to analyse the data. [4*, 54, 55] As a minimum standard, authors should explicitly state whether active or passive surveillance systems were used. [46, 49]
RCTs published in journals with a higher impact factor, in which spinal manipulation was delivered by a chiropractor and to multiple/unclear regions, were more likely to report on adverse events. While it is perhaps intuitive that better designed studies, that is, those at a lower risk of bias, could reasonably be published in higher impact journals, this does not appear to be the case as there was no influence of risk of bias level in the final model. This disconnect between the publication of studies with better methodological quality in higher impact journals is also seen in the medical literature. Specifically, a previous study reported that there were methodological weaknesses in 184 studies published in 2015–2016 by 4 of the top ranked general medical journals (BMJ, JAMA, Lancet and NEJM). [54] Furthermore, while there is no obvious reason why studies in which spinal manipulation was delivered by a chiropractor would be more likely to report on adverse events, possible reasons for this finding could include that chiropractors are more likely to deliver cervical spine manipulation in general and/or that due to perceived ‘risks’ of cervical spine manipulation, other professions choose not to conduct trials investigating this intervention. This hypothesis is suggested by the data which shows that while not achieving statistical significance, studies in which cervical spine manipulation was delivered had approximately three times greater odds of reporting on adverse events. It is possible that this result did not achieve statistical significance due to the relatively small number of studies reporting on manipulation delivered only to the cervical spine. Regarding the increased likelihood of studies reporting on adverse events if spinal manipulation was delivered to multiple/unclear regions, it is possible that this finding is spurious as there was a larger number of studies (n=49) in this category compared with studies in which the intervention was delivered to a single region. This hypothesis is supported by a secondary analysis of our previous review which reported that the region treated was not a significant predictor for reporting on adverse events. [56]
Due to the methodological design of the review, we are unable to comment on the incidence of adverse events associated with spinal manipulation. Furthermore, RCTs are not necessarily the best research design for collecting data on serious adverse events as they often have strict inclusion criteria and may exclude participants who are at risk of experiencing such events. Additionally, RCTs are powered to detect intervention effects and thus are likely to be underpowered for estimating the risk of serious adverse events. Despite this, the consistent reporting of the number of spinal manipulations delivered to every participant in RCTs could allow for the calculation of accurate incidence rates for all classifications of adverse events (serious included) and could eventually facilitate the pooling of data across multiple studies thus allowing for a better informed risk-benefit assessment of spinal manipulation. [25, 46] We acknowledge that the calculation of accurate incidence rates is not straight-forward. Indeed, factors such as the use of different spinal manipulation techniques, how to parse out adverse events attributable to different interventions (eg, orthopaedic testing, soft tissue treatment or exercise) and how to amalgamate reports on different cohorts (eg, neck vs low back pain) must all be considered. While this task seems insurmountable, consistent reporting of the number of spinal manipulations delivered to every participant in RCTs is the first step towards this goal. To this end, the number of spinal manipulations delivered was only available in 75 (48.7%) of the included studies. Coupled with the implementation of standardised definitions and classification systems for adverse events associated with spinal manipulation, reporting on the number of spinal manipulations delivered in each study could allow for the interdisciplinary calculation of incidence rates for all classifications across all healthcare professionals delivering the intervention. Such an outcome is extremely important in the context of obtaining informed consent to deliver spinal manipulation. Specifically, in many countries in which spinal manipulation is delivered, the process of obtaining informed consent requires the disclosure of all material information that a reasonable patient would require to make an informed decision about whether or not to receive that intervention. [57] In the absence of accurate incidence rates for the different classifications of adverse events associated with spinal manipulation, this is a difficult task for the clinician to perform.
There are several differences between the current review and our 2016 review.26 Specifically, the current review included an improved search strategy, including both an expansion to the number of databases searched (ie, MEDLINE (Ovid), Embase, CINAHL and ICL were added) in addition to the inclusion of several search terms that did not limit the search to spinal manipulation delivered by chiropractors and osteopaths (ie, physiotherapists, naprapaths and medical manipulation were added). Additionally, the current review reports on analyses that we had previously reported separately in two manuscripts: the original review [26] and a secondary analysis. [56] By reporting these analyses in a single manuscript, we hope it is clearer for readers to identify that the current level of reporting of adverse events associated with spinal manipulation in RCTs is both poor and not consistent with established standards, and understand the possible explanations for this observation. By streamlining the dissemination of this information, we hope to make it easier for readers to identify areas in which researchers may improve the reporting of adverse events in this field.
Limitations
There are several limitations to this literature review. First, the decision to classify the reporting of adverse events as ‘direct’ (explicit description of operational definition of an adverse event provided and/or how data on adverse events were measured and/or a substantial description of adverse events observed during data collection provided) as opposed to ‘indirect’ (no explicit reporting of such information) was arbitrary. However, this classification did not influence whether the study reported on adverse events or not. As such, we do not feel this factor had any material influence on our results. Second, as outlined above, small differences in the methodology between the current and previous reviews [26, 56] mean that it is not possible to directly compare all reported findings between the two reviews. However, as these differences occurred due to methodological improvements in the current review, we do not believe this affected the results and/or discussion in the current review.
Conclusion
While the current level of reporting of adverse events associated with spinal manipulation in RCTs has increased since our 2016 publication on the same topic, the level remains low and inconsistent with established standards. As such, it is imperative for authors, journal editors and administrators of clinical trial registries to ensure there is more balanced reporting of both benefits and harms of spinal manipulation in RCTs. We strongly recommend that authors adhere to the most recent CONSORT Harms checklist when reporting their results and advocate for the creation of standardised definitions and classification systems relating to adverse events in manual therapy. This will facilitate the future pooling of adverse events data across all professions sing spinal manipulation and improve the ability to calculate incidence rates for the different levels of adverse events.
Supplementary Material
Reviewer Comments (308K, pdf)
Draft Revisions (2.4M, pdf)Acknowledgements
The authors would like to acknowledge Dr Martina Gosteli for her assistance with the literature search.
Contributors:
LMG: conceptualisation, screening, risk of bias assessment, data extraction and curation, formal analysis, methodology, project administration, visualisation and writing—original draft, review and editing and guarantor.
RPL: data extraction and curation, formal analysis, methodology, visualisation and writing—original draft, review and editing. BTB: screening, risk of bias assessment and writing—review and editing.
RE: screening, risk of bias assessment, methodology and writing—review and editing.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
References:
Whalen W, Farabaugh RJ, Hawk C, et al.
Best-Practice Recommendations for Chiropractic Management
of Patients With Neck Pain
J Manipulative Physiol Ther. 2019 (Nov); 42 (9): 635–650Bussieres AE, Stewart G, Al-Zoubi F, Decina P, Descarreaux M, Haskett D, Hincapie C, et al.
Spinal Manipulative Therapy and Other Conservative Treatments
for Low Back Pain: A Guideline From the Canadian
Chiropractic Guideline Initiative
J Manipulative Physiol Ther. 2018 (May); 41 (4): 265–293Bussières, AE, Stewart, G, Al Zoubi, F et al.
The Treatment of Neck Pain-Associated Disorders and
Whiplash-Associated Disorders: Clinical Practice Guideline
J Manipulative Physiol Ther. 2016 (Oct); 39 (8): 523–564Oliveira CB, Maher CG, Pinto RZ, et al..
Clinical practice guidelines for the management of non-specific
low back pain in primary care: an updated overview.
Eur Spine J 2018;27:2791–803.
10.1007/s00586-018-5673-2Beliveau PJH, Wong JJ, Sutton DA, Simon NB, Bussieres AE, Mior SA, et al.
The Chiropractic Profession: A Scoping Review of Utilization Rates,
Reasons for Seeking Care, Patient Profiles, and Care Provided
Chiropractic & Manual Therapies 2017 (Nov 22); 25: 35Lin I, Wiles L, Waller R, et al.
What Does Best Practice Care for Musculoskeletal Pain Look Like?
Eleven Consistent Recommendations From High-quality
Clinical Practice Guidelines: Systematic Review
British J Sports Medicine 2020 (Jan); 54 (2): 79–86National Institute for Health and Care Excellence (NICE):
Low Back Pain and Sciatica in Over 16s:
Assessment and Management (PDF)
NICE Guideline, No. 59 2016 (Nov): 1–1067Puentedura EJ, March J, Anders J, et al..
Safety of cervical spine manipulation: are adverse events preventable
and are manipulations being performed appropriately?
A review of 134 case reports.
J Man Manip Ther 2012;20:66–74.
10.1179/2042618611Y.0000000022Biller J, Sacco RL, Albuquerque FC, et al.:
Cervical Arterial Dissections and Association With Cervical
Manipulative Therapy A Statement for Healthcare Professionals
From the American Heart Association/ American Stroke Association
Stroke. 2014 (Oct); 45 (10): 3155–3174Funabashi M, Pohlman KA, Goldsworthy R, et al..
Beliefs, perceptions and practices of chiropractors and patients about
mitigation strategies for benign adverse events after spinal manipulation therapy.
Chiropr Man Therap 2020;28.
10.1186/s12998-020-00336-3Heneghan NR, Davies SE, Puentedura EJ, et al..
Knowledge and pre-thoracic spinal thrust manipulation examination:
a survey of current practice in the UK.
J Man Manip Ther 2018;26:301–9.
10.1080/10669817.2018.1507269Albuquerque FC, Hu YC, Dashti SR, et al..
Craniocervical arterial dissections as sequelae of
chiropractic manipulation: patterns of injury and management.
JNS 2011;115:1197–205. 10.3171/2011.8.JNS111212Ernst E.
Deaths after chiropractic: a review of published cases.
Int J Clin Pract 2010;64:1162–5.
10.1111/j.1742-1241.2010.02352.xChurch EW, Sieg EP, Zalatimo O, Hussain NS, Glantz M, Harbaugh RE.
Systematic Review and Meta-analysis of Chiropractic Care and
Cervical Artery Dissection: No Evidence for Causation
Cureus 2016 (Feb 16); 8 (2): e498Cassidy JD, Boyle E, Cote P, et al.
Risk of Vertebrobasilar Stroke and Chiropractic Care: Results of
a Population-based Case-control and Case-crossover Study
Spine (Phila Pa 1976) 2008 (Feb 15); 33 (4 Suppl): S176–183Whedon, JM, Song, Y, Mackenzie, TA, Phillips, RB, Lukovits, TG, and Lurie, JD.
Risk of Stroke After Chiropractic Spinal Manipulation in Medicare B
Beneficiaries Aged 66 to 99 Years With Neck Pain
J Manipulative Physiol Ther. 2015 (Feb); 38 (2): 93–101J.D. Cassidy, E. Boyle, P. Cote, S. Hogg-Johnson, S.J. Bondy, S. Haldeman
Risk of Carotid Stroke after Chiropractic Care:
A Population-Based Case-Crossover Study
J Stroke Cerebrovasc Dis. 2017 (Apr); 26 (4): 842–850Whedon JM, Petersen CL, Li Z, et al.
Association Between Cervical Artery Dissection and Spinal
Manipulative Therapy - A Medicare Claims Analysis
BMC Geriatrics 2022 (Nov 29); 22 (1): 917Gorrell LM, Kuntze G, Ronsky JL, et al.
Kinematics of the Head and Associated Vertebral Artery Length Changes
During High-velocity, Low-amplitude Cervical Spine Manipulation
Chiropractic & Manual Therapies 2022 (Jun 1); 30 (1): 28Gorrell LM, Sawatsky A, Edwards WB, et al..
Vertebral arteries do not experience tensile force during
manual cervical spine manipulation applied to human cadavers.
J Man Manip Ther 2022:1–9.
10.1080/10669817.2022.2148048Begg C, Cho M, Eastwood S, et al..
Improving the quality of reporting of randomized controlled trials.
The CONSORT statement.
JAMA 1996;276:637–9.
10.1001/jama.276.8.637Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191–4.
Schulz KF, Altman DG, Moher D, et al.
Consort 2010 statement: updated guidelines for
reporting parallel group randomised trials.
BMC Med 2010;8:18.
10.1186/1741-7015-8-18Ioannidis JPA, Evans SJW, Gøtzsche PC, et al.
Better reporting of harms in randomized trials: an extension of the CONSORT statement.
Ann Intern Med 2004;141:781–8.
10.7326/0003-4819-141-10-200411160-00009Junqueira DR, Phillips R, Zorzela L, et al.
Time to improve the reporting of harms in randomized controlled trials.
J Clin Epidemiol 2021;136:216–20.
10.1016/j.jclinepi.2021.04.020Gorrell LM, Engel RM, Brown B, et al.
The reporting of adverse events following spinal manipulation
in randomized clinical trials-a systematic review.
Spine J 2016;16:1143–51.
10.1016/j.spinee.2016.05.018Page MJ, McKenzie JE, Bossuyt PM, et al.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
BMJ 2021;372:71. 10.1136/bmj.n71Herzog W:
The Biomechanics of Spinal Manipulation
J Bodyw Mov Ther. 2010 (Jul); 14 (3): 280–286Bergmann T.
Chiropractic Technique Principles and Procedures, 3rd ed.
Missouri: USA, 2011.Pohlman KA, Beirne MO, Thiel H, Cassidy JD, Mior S, Hurwitz EL, et al.
Development and Validation of Providers’ and Patients’
Measurement Instruments to Evaluate Adverse Events
After Spinal Manipulation Therapy
Eur J Integr Med. 2014 (Aug); 6 (4): 451–466Walker, BF, Hebert, JJ, Stomski, NJ et al.
Outcomes of Usual Chiropractic.
The OUCH Randomized Controlled Trial of Adverse Events
Spine (Phila Pa 1976). 2013 (Sep 15); 38 (20): 1723–1729Carnes D, Mullinger B, Underwood M.
Defining adverse events in manual therapies:
a modified Delphi consensus study.
Man Ther 2010;15:2–6.
10.1016/j.math.2009.02.003Dunning J, Butts R, Zacharko N, et al.
Corrigendum to "spinal manipulation and perineural electrical dry needling
in patients with cervicogenic headache: a multi-center randomized clinical trial"
[the spine Journal 21/2 (2021) p284-295].
Spine J 2021;21:1238.
10.1016/j.spinee.2021.04.014Maiers M, Hartvigsen J, Evans R, et al.
Short- or long-term treatment of spinal disability in
older adults with manipulation and exercise.
Arthritis Care Res (Hoboken) 2019;71:1516–24.
10.1002/acr.23798Vining R, Long CR, Minkalis A, Gudavalli MR, Xia T, Walter J, et al.
Effects of Chiropractic Care on Strength, Balance, and Endurance
in Active-Duty U.S. Military Personnel with Low Back Pain:
A Randomized Controlled Trial
J Altern Complement Med 2020 (Jul); 26 (7): 592–601–693Schulz C, Evans R, Maiers M, Schulz K, Leininger B, Bronfort G (2019)
Spinal Manipulative Therapy and Exercise for Older Adults
with Chronic Low Back Pain: A Randomized Clinical Trial
Chiropractic & Manual Therapies 2019 (May 15); 27: 21Ouzzani M, Hammady H, Fedorowicz Z, et al.
Rayyan-a web and mobile APP for systematic reviews.
Syst Rev 2016;5:210.
10.1186/s13643-016-0384-4International Committeee of Medical Journal Editors (ICMJE) .
Journals following the ICMJE recommendations; 2016.Clarivate Journal citation reports. Available:
https://clarivate.com/webofsciencegroup/solutions/journal-citation-reports/
[Accessed 29 May 2022].Higgins JP, Savovi J, Page MJ, et al.
Assessing risk of bias in a randomized trial.
In: Cochrane Handbook for Systematic Reviews of Interventions.
John Wiley & Sons, Ltd, 2019: 205–28.Funabashi M, Pohlman KA, Gorrell LM, et al.
Expert Consensus on a Standardised Definition and Severity Classification
for Adverse Events Associated with Spinal and Peripheral Joint Manipulation
and Mobilisation: Protocol for an International E-Delphi Study
BMJ Open 2021 (Nov 11); 11 (11): e050219Carnes D, Mars TS, Mullinger B, et al.
Adverse events and manual therapy: a systematic review.
Man Ther 2010;15:355–63.
10.1016/j.math.2009.12.006Carlesso LC, Macdermid JC, Santaguida LP.
Standardization of adverse event terminology and reporting in
orthopaedic physical therapy: application to the cervical spine.
J Orthop Sports Phys Ther 2010;40:455–63.
10.2519/jospt.2010.3229Carlesso LC, Cairney J, Dolovich L, et al.
Defining adverse events in manual therapy: an exploratory
qualitative analysis of the patient perspective.
Man Ther 2011;16:440–6.
10.1016/j.math.2011.02.001Carlesso LC, Gross AR, Santaguida PL, et al.
Adverse events associated with the use of cervical manipulation and
mobilization for the treatment of neck pain in adults:
a systematic review.
Man Ther 2010;15:434–44.
10.1016/j.math.2010.02.006Zorzela L, Loke YK, Ioannidis JP, et al.
PRISMA harms checklist: improving harms reporting in systematic reviews.
BMJ 2016;352:i157. 10.1136/bmj.i157Funabashi M, Gorrell LM, Pohlman KA, et al.
Definition and Classification for Adverse Events Following
Spinal and Peripheral Joint Manipulation and Mobilization:
A Scoping Review
PLoS One 2022 (Jul 15); 17 (7): e0270671Pitkin RM.
The importance of the Abstract.
Obstet Gynecol 1987;70:267.Zorzela L, Golder S, Liu Y, et al.
Quality of reporting in systematic reviews of adverse events: systematic review.
BMJ 2014;348:f7668. 10.1136/bmj.f7668Komorowski AS, MacKay HJ, Pezo RC.
Quality of adverse event reporting in phase III randomized controlled trials
of breast and colorectal cancer: a systematic review.
Cancer Med 2020;9:5035–50. 10.1002/cam4.3095Berwanger O, Ribeiro RA, Finkelsztejn A, et al.
The quality of reporting of trial Abstracts is suboptimal:
survey of major general medical journals.
J Clin Epidemiol 2009;62:387–92.
10.1016/j.jclinepi.2008.05.013Pitrou I, Boutron I, Ahmad N, et al.
Reporting of safety results in published reports of randomized controlled trials.
Arch Intern Med 2009;169:1756–61.
10.1001/archinternmed.2009.306Nuovo J, Sather C.
Reporting adverse events in randomized controlled trials.
Pharmacoepidemiol Drug Saf 2007;16:349–51.
10.1002/pds.1310Phillips R, Hazell L, Sauzet O, et al.
Analysis and reporting of adverse events in randomised controlled trials: a review.
BMJ Open 2019;9:e024537.
10.1136/bmjopen-2018-024537Phillips R, Sauzet O, Cornelius V.
Statistical methods for the analysis of adverse event data in
randomised controlled trials: a scoping review and taxonomy.
BMC Med Res Methodol 2020;20:288.
10.1186/s12874-020-01167-9Gorrell LM, Brown B, Lystad RP, et al.
Predictive factors for reporting adverse events following spinal manipulation
in randomized clinical trials-secondary analysis of a systematic review.
Musculoskelet Sci Pract 2017;30:34–41.
10.1016/j.msksp.2017.05.002Winterbottom M, Boon H, Mior S, et al.
Informed consent for chiropractic care:
comparing patients’ perceptions to the legal perspective.
Man Ther 2015;20:463–8.
10.1016/j.math.2014.11.009
Return to ADVERSE EVENTS
Since 5-15-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |