

Mechanisms and Effects of Spinal High-velocity,
Low-amplitude Thrust Manipulation:
Previous TheoriesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther 2002 (May); 25 (4): 251–262 ~ FULL TEXT
David W. Evans, BSc (Hons) Osta
British School of Osteopathy,
London, United Kingdom
OBJECTIVES: When the clinical efficacy of spinal manipulative treatment for spinal pain has been assessed, high-velocity low-amplitude thrust (HVLAT) manipulation and mobilization have been regarded as clinical interventions giving identical and equivalent biologic effects. The objective of this review is to critically discuss previous theories and research of spinal HVLAT manipulation, highlighting reported neurophysiologic effects that seem to be uniquely associated with cavitation of synovial fluid.
DATA SOURCE: The biomedical literature was searched for research and reviews on spinal manipulation. MEDLINE and EMBASE databases were used to help find relevant articles.
STUDY SELECTION: All articles relevant to the objectives were selected.
DATA EXTRACTION: All available data were used.
DATA SYNTHESIS: The main hypotheses for lesions that respond to HVLAT manipulation were critically discussed:(1) release of entrapped synovial folds or plica,
(2) relaxation of hypertonic muscle by sudden stretching,
(3) disruption of articular or periarticular adhesions, and
(4) unbuckling of motion segments that have undergone disproportionate displacements.RESULTS: There appear to be 2 separate modes of action from zygapophyseal HVLAT manipulation. Intra-articular "mechanical" effects of zygapophyseal HVLAT manipulation seem to be absolutely separate from and irrelevant to the occurrence of reported "neurophysiologic" effects. Cavitation should not be an absolute requirement for the mechanical effects to occur but may be a reliable indicator for successful joint gapping.
CONCLUSIONS: It is hoped that identification of these unique neurophysiologic effects will provide enough theoretical reason for HVLAT manipulation and mobilization to be assessed independently as individual clinical interventions.
From the FULL TEXT Article
Introduction
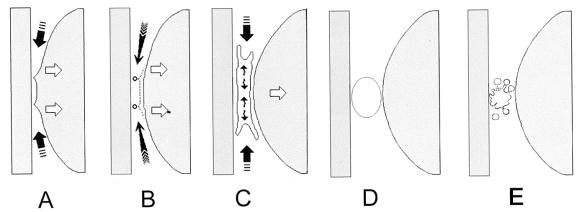
Spinal manipulation has been used for more than 2000 years. [1] There have been many attempts to explain the physiology of the various effects of spinal manipulation, particularly those of the high-velocity low-amplitude thrust (HVLAT or HVT)type. As its name suggests, this type of manipulation uses a high velocity “impulse” or “thrust” which is applied to a diarthrodial synovial joint over a very short amplitude. This type of manipulation is usually associated with an audible “crack,” which is often viewed as signifying a successful manipulation. [2] The cracking sound is caused by an event termed “cavitation,” occurring within the synovial fluid (SF) of the joint (Figure 1).
Figure 1. Cavitation. Schematic representation of surface geometry and shapes of growing cavities at a high separation speed (v > > vc as is likely with HVLAT manipulation) where doughnut (toroidal)-shaped cavities form around, rather than at the center, of the contact zone.
A, During separation, the outer regions of the circular contact zone become pointed. This deformation occurs because at this speed, the central region of the contact zone separates, whereas the outer region remains almost unmoved, creating a circular rim.
B, Surfaces snap back at the circular rim where the cavity initially forms.
C, Coalescence of toroid into single dendritic cavity that grows to reach a maximum bubble size.
D, The newly formed spherical bubble reaches its maximum size.
E, Because of its instability, the single bubble collapses to form a “cloud” of many smaller bubbles (demonstrable by radiography as a radiolucent region), which later shrink as the gas and vapor dissolve (see later).Adapted from Chen YL, Kuhl T, Israelachvili J.
Mechanism of cavitation damage in thin liquid films: collapse damage vs. inception damage.
Wear 1992; 153: 31–51.
Reproduced with permission from Elsevier Science.
Cavitation is the term used to describe the formation and activity of bubbles (or cavities) within fluid through local reduction in pressure. [3, 4] Although there is strong evidence for the clinical efficacy of spinal manipulative therapy for both acute and chronic low back pain, [5, 6] the physiological mechanisms behind these clinical effects are not yet clear. [7] This paucity of basic knowledge has led to the grouping of spinal HVLAT manipulation and mobilization (a gentle, often oscillatory, passive movement) together as 1 intervention when previously scrutinized for efficacy. [5, 6] The purpose of this review is to highlight some of the unique effects that are seen with spinal HVLAT manipulation, particularly those that seem only to occur in association with the cavitation event. This may help to provide enough theoretical reason to assess mobilization and manipulation as separate clinical entities.
Discussion
Previous theories
In a brief review, Shekelle [8] states, “There are four main hypotheses for lesions that respond to (HVLAT) manipulation:(1) release of entrapped synovial folds or plica,
(2) relaxation of hypertonic muscle by sudden stretching,
(3) disruption of articular or periarticular adhesions, and
(4) unbuckling of motion segments that have undergone disproportionate displacements.”These “main hypotheses” will be discussed.
Release of entrapped synovial folds or plica
Intra-articular formations have been identified throughout the vertebral column. [9] Giles and Taylor [10] demonstrated by light and transmission electron microscopy the presence of nerve fibers (0.6 to 1 mm in diameter) coursing through synovial folds, remote from blood vessels, that were most likely nociceptive. They concluded, “Should the synovial folds become pinched between the articulating facet surfaces of the zygapophyseal joint, the small nerves demonstrated in this study may have clinical importance as a source of low back pain.” Bogduk and Jull [11] reviewed the likelihood of intra-articular entrapments within zygapophyseal joints as potential sources of pain, leading to an “acute locked back.” They commented that the theory of entrapment of tissue, causing pain as a result of “pinching,” “demands that the joint be in, or near to, a neutral position, for only in that position are the articular surfaces sufficiently apposed to trap a meniscus (or synovial fold). Consequently, this dictates that the locked position assumed by the patient is near to neutral, but the clinical features of an acute locked back are that the patient is locked in a flexion position, unable to extend.” Synovial tissue entrapment is therefore unlikely to be the cause of an “acute locked back” of this type but may be the cause of other more transient “pinching” conditions.
Fibro-adipose meniscoids have also been identified as structures capable of creating a painful situation. [11, 12] Bogduk and Jull [11] reviewed the possible role of fibro-adipose meniscoids causing pain purely by creating a tractioning effect on the zygapophyseal joint capsule, again after intra-articular pinching of tissue. They argued that it was unlikely that the meniscoids would be sufficiently strong to distort the zygapophyseal joint capsule. The basal segment of the meniscoid consists only of adipose tissue and synovium, and “traction exerted through such tissues would tend to rupture them or cleave them from the joint capsule, rather than be transmitted in full force to the joint capsule.”
The theory of a meniscoid entrapment would also be an unlikely cause of an “acute locked back” because of the prerequisite of a neutral position, as discussed previously. Bogduk and Jull [11] instead proposed that on flexion of the lumbar spine, the inferior articular process of a zygapophyseal joint moves upward, taking a meniscoid with it. On attempted extension, the inferior articular process returns toward its neutral position, but instead of re-entering the joint cavity, the meniscoid impacts against the edge of the articular cartilage and buckles, forming a space-occupying “lesion” under the capsule: a meniscoid extrapment (Fig 2).
A large number of type III and type IV nerve fibers (nociceptors) have been observed within capsules of zygapophyseal joints. [10, 13, 14] Pain occurs as distension of the joint capsule provides a sufficient stimulus for these nociceptors to depolarize. Muscle spasm would then occur to prevent impaction of the meniscoid. The patient would tend to be more comfortable with the spine maintained in a flexed position, because this will disengage the meniscoid. Extension would therefore tend to be inhibited. This condition has also been termed a “joint lock” or “facet-lock,” the latter of which indicates the involvement of the zygapophyseal joint.
The presence of fibro-adipose meniscoids in the cervical zygapophyseal joints [12, 15] suggests that a similar phenomenon might occur, but in the neck the precipitating movement would be excessive rotation. The clinical features of cervical meniscoid extrapment would be those of an acute torticollis in which attempted derotation would cause impaction and buckling of the extrapped meniscoid and painful capsular strain. Muscle spasm would then occur to prevent impaction of the meniscoid by keeping the neck in a rotated position. Under these circumstances the muscle spasm would not be the primary cause of torticollis but a secondary reaction to the extrapment of the meniscoid. [12]
An HVLAT manipulation, involving gapping of the zygapophyseal joint, [16] reduces the impaction and opens the joint, so encouraging the meniscoid to return to its normal anatomic position in the joint cavity. This ceases the distension of the joint capsule, thus reducing pain [11] (Fig 2).
It is noteworthy that the International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” [17] This is significant because distension of a zygapophyseal capsule can cause pain without any actual tissue damage, a characteristic of most episodes of nonspecific back pain. [18]
The onset of an acute locked back (or neck) is commonly of little or no trauma, [19] such as bending to pick up a newspaper or turning over during sleep. Because these conditions are unlikely to involve any significant tissue damage, pain may be due only to potential damage. Nontraumatic onset such as that described previously should therefore not be a contraindication for a high-velocity procedure. On the contrary, in the absence of “red flags” for physical risk factors, [20, 21] a nontraumatic onset should provide a good indication for a favorable outcome from an HVLAT manipulation, even in a very painful and acute state. This argument merits further investigation.
Zygapophyseal joint gapping induced during an HVLAT manipulation would further stretch the highly innervated joint capsule, leading to a “protective” reflex muscular contraction, as shown in electromyograpic studies. [22–30] The most important characteristic of a manipulative procedure that will provide joint gapping, before the induction of protective reflex muscular contraction, would be high velocity. Brennan et al [31] found that the thrusting phase of an HVLAT manipulation required 91 ± 20 ms to develop the peak force. If this period is compared with the time delay between the onset of the thrusting force and the onset of electromyographic activity, which ranges from 50 to 200 ms, [24] we can see that a force of sufficient magnitude to gap the joint can be applied in a shorter time than that required for the initiation of a mechanoreceptor-mediated muscular reflex. Furthermore, once the muscle is activated (ie, there is an electromyographic signal), it will take approximately another 40 to 100 ms until the onset of muscular force. It therefore seems unlikely that there are substantial muscular forces resisting the thrusting phase of HVLAT manipulation. [24] Thus HVLAT manipulation would again appear to be the treatment of choice for a meniscoid extrapment.
The cavitation event may not be a prerequisite for a “successful” HVLAT manipulation in the case of a meniscoid extrapment and may be an incidental side effect of high-velocity zygapophyseal joint gapping (which would be a prerequisite for success). Audible indication of successful joint gapping may, however, be regarded as desirable in itself as a clinical measure of “success.” A clinician's perception of the occurrence of cavitation during an HVLAT manipulation has been shown to be very accurate [26] and would therefore be a reliable measure of a “successful” joint gapping.
Gainsbury [32] claims that these “acute locked joints” often occur in joints with a history of “hypermobility.” However, there is no clinical evidence to validate this statement, and it is therefore only speculative. In theory, increased segmental mobility, or more accurately, decreased active control of intersegmental motion around the “neutral zone” (the motion region of the joint where the passive osteo-ligamentous stability mechanisms exert little or no influence [33, 34]) would be a logical predisposing factor for zygapophyseal meniscal extrapment. Consequently, symptom-relieving HVLAT manipulation will not provide a lasting solution to this segmental instability, and a segmental stabilization exercise regimen should form the basis of rehabilitation [35] in an attempt to avoid future recurrence.
Although meniscoid replacement may be the mechanism by which pain relief would be achieved from zygapophyseal HVLAT manipulation in an “acute locked back,” it does not explain reported nonmechanical effects that are associated with HVLAT manipulations (see following text).
Relaxation of hypertonic muscle by sudden stretching
Viscoelasticity is a property of soft tissues whereby the strain induced in the tissue is dependent on the rate of loading of the applied stress. [36] The connective tissue in and around the muscle belly, tendons, joint capsules, and ligaments all exhibit viscoelastic behavior under loading. [37–39] This is thought to be due to biochemical interaction between the collagen fibers and the ground substance. [40] When ligament and tendon specimens are subjected to increased strain rates (loading rates), the linear portion of the stress-strain curve becomes steeper, indicating greater stiffness of the tissue at higher strain rates. [41–43] This demonstrates that the faster the rate of loading is, the more energy is “stored” within the tissue, allowing it to withstand larger forces when the force is delivered more rapidly. This property is a valuable attribute in tendons [44] but would also imply that rapid loading would not cause an efficient mechanical stretch of a “hypertonic” muscle and would be more likely to damage musculotendinous structures in a similar way to “ballistic” type stretching. [45] Efficient stretching should be of a very low velocity, or at a constant force to cause “creep,” and should be maintained for at least 12 to 18 seconds. [39, 46]
The forces produced during HVLAT manipulation of a zygapophyseal joint can be relatively large. [47] However, if applied properly, these forces should not be significantly transferred to the soft tissues and should be predominantly dissipated within the SF, which also has viscoelastic properties, [48–53] for cavitation to occur. [54] Watson et al [53] showed that the “pulse area” parameter from an accelerometer measuring cavitation (a measure of kinetic energy imparted to the accelerometer) was highly correlated with the “drop in load” (resistance to distraction force) of a joint. Thus the synovial fluid absorbs much of the kinetic energy required to cause cavitation.
Mechanoreceptors, proprioceptors, and even nociceptive afferents of both joint capsules and musculotendinous structures have long been viewed as the probable “gateway” through which the nervous system (and consequently motor “tone”) would be influenced by HVLAT manipulation. [25, 30, 55–64] Avramovet al [65] have shown that loading of the zygapophyseal joint excites 3 patterns of nerve discharges: short-duration bursts during change in loading, prolonged discharges at low load levels, and prolonged discharges at high load levels, indicating that different units in the joint capsule have different levels of stress thresholds. It seems logical, however, that the receptor populations that are activated on passive high load would be present in a protective role and would tend to activate rather than relax or inhibit protective muscular tissue. This is especially so, because it has been previously suggested that if forces involved with a spinal HVLAT manipulation [22, 47, 66, 67] were transferred to the surrounding joint capsule and soft tissues, nociceptive afferents would be activated. [57, 58] Lederman [68] states, “It has long been believed that manual techniques such as high-velocity thrusts or adjustments can normalize abnormal motor tone. The reduced motor tone is attributed to the stimulation of inhibitory afferents by manipulation. This is highly unlikely as sudden stretch produced by this form of manipulation will excite rather than inhibit the motor neuron.”
The protective role of the musculature against potentially harmful force to joints, by way of reflex arcs, creates synergism between the passive (capsuloligamentous) and active (muscular) joint restraints. These have been studied in various animal and human joints. [30, 69–73] Wyke [30] distracted the zygapophyseal joint between C3 and C4 in anesthetized cats and measured an increased electromyographic response associated with such distractions in the neck and limb musculature. He concluded from this experiment that joint capsule mechanoreceptors will influence (most likely through reflex pathways) the activation of the neck and limb musculature. These experiments indicated that an excitatory stimulus rather than an inhibitory (relaxatory) stimulus was given to these muscles after stress placed through their related joint capsules. Because of the rich innervation of human zygapophyseal joint capsules, [13, 14, 30, 62, 74] it would be reasonable to suggest that a similar synergistic relationship between the capsular and ligamentous structures and paraspinal muscles occurs also in humans.
Observations made by Herzog et al [22–24, 26–29] confirm this. They observed that HVLAT manipulation caused (excitatory) reflex responses in the back and limb musculature. They decided that the observed reflex responses could have a variety of origins (“the external force, the rate of force application, the impulse, the cavitation, etc”) and therefore compared the reflex responses of HVLAT manipulation treatments that resulted in cavitation of the joint (as judged by the treating chiropractor and confirmed by accelerometer measurements) with corresponding treatments that did not result in cavitation. [26] They concluded, “the reflex responses seemed to be the same in both cases, which suggests that cavitation was not needed to elicit the observed reflex responses.” They further attempted to evaluate the role of cavitation in eliciting reflex responses by measuring reflex responses associated with low-velocity “treatments” (ie, treatments in which the point and direction of force application were identical to the previous treatments; however, the peak forces were reached within approximately 2 to 3 seconds rather than 100 to 150 ms). These slow treatments never resulted in measurable reflex responses, regardless of whether cavitation was elicited. [29] Thus they concluded, “Neither the cavitation event nor the magnitude of the applied force caused the reflex activation of the back musculature.” High-velocity movement alone seemed to activate the response, indicating that the reflex muscular contractions measured by electromyography were mediated by mechanoreceptor afferents in the joint capsules and muscles.
There is recent evidence that both lumbar spine mobilization and manipulation result in significant transient attenuation of alpha motor neuronal activity, as measured by the amplitude of the extremely sensitive gastrocnemius Hoffmann reflex (H-reflex or H-wave), with a return to baseline values exhibited 30 seconds after intervention. [75] Cavitation was also an unimportant component of this temporary effect. However, it is quite likely that this transient H-wave attenuation is simply a latent artefact of the mechanoreceptor-mediated excitatory muscular reflex response and therefore has equivocal clinical relevance.
Herzog et al [27] came to the logical conclusion that “an audible release does not (by itself) evoke muscle activation or a joint proprioceptive reflex response as has been speculated in the literature.” This conclusion can be taken in 2 directions. If the excitatory muscular reflex response (and consequential H-wave attenuation) was taken as an important requisite for the beneficial effects of an HVLAT manipulation, then the cavitation event would be unimportant. However, it could also be argued that these muscular reflex responses are incidental to any potentially beneficial effects achieved from an HVLAT manipulation, and the cavitation event alone produces responses not observable by electromyography (see following text).
A rapid “sudden stretch” seems unlikely to produce a clinically beneficial and lasting neurophysiologic relaxation of hypertonic muscles. Therefore, if an HVLAT manipulation has a lasting modifying effect on the tone or “irritability” of muscles associated with the joint, the nervous system is being influenced in some other way. If cavitation was an unimportant element of a spinal HVLAT manipulation and it was only mechanoreceptor stimulation that created the beneficial effects, it should follow that continual repetitive HVLAT manipulations immediately after the initial cavitation-producing thrust (ie, within the 15– to 20–minute “refractory period” during which further cavitation is not possible) would create cumulative beneficial effects. From clinical experience, this is clearly not the case but requires further investigation.
The term “relaxation of hypertonic muscle” [8] implies that there is a reduction of alpha-motorneurone excitability or activity to innervated muscle. Facilitation of some motor reflexes, however, is independent of changes in the excitability of afferent terminals in the dorsal horn and of motorneurones. A more likely explanation for the behavior of this “hypertonic muscle” is that its innervation is mediated by sensitized spinal interneurones. [76] Muscles innervated by a sensitized interneurone population, eliciting properties of secondary hyperalgesia (sometimes described in terms of “myofascial trigger points” [77, 78]) have been associated with very small loci of spontaneous electromyographic activity. [79, 80] These hyperalgesic regions seem also to be associated with a palpable “taut band,” which gives increased muscle “tone.” [81]
After zygapophyseal HVLAT manipulation, reductions in paraspinal spontaneous electromyographic activity [82, 83] and reduced hyperalgesia of paraspinal myofascial trigger points [84] have been demonstrated. This finding seems to indicate that any observable “motor” effects that HVLAT manipulation has may be mediated by alterations in central sensitization of the dorsal horn.
Regarding alterations in central sensitization, it has also been demonstrated that zygapophyseal HVLAT manipulation caused not only a reduction of paraspinal hyperalgesia in subjects with symptoms [84–86] but also an increase in paraspinal pain thresholds to a noxious stimulus in subjects with no symptoms. [87]
Thus it seems more appropriate to describe one of the neurophysiologic effects of zygapophyseal HVLAT manipulation as creating “hypoalgesia” (of the dorsal horn associated with) the spinal segment manipulated, [88] rather than “relaxation of hypertonic muscle,” whatever the mechanism by which this is achieved.
In a series of studies, Brennan et al [31, 89–92] investigated the effect of spinal HVLAT manipulation causing cavitation (“sufficient to produce an auditory release or palpable joint movement”) on cells of the immune system. They found that a single manipulation to either the thoracic or lumbar spine resulted in a short-term priming of polymorphonuclear neutrophils to respond to an in vitro particulate challenge with an enhanced respiratory burst (RB) as measured by chemiluminescence in subjects with and without symptoms. The enhanced RB was accompanied by a two-fold rise in plasma levels of the neuropeptide substance P (SP).
SP is an 11-amino acid polypeptide and is one of a group of neuropeptides known as tachykinins. These are peptides that are produced in the dorsal root ganglion (DRG) and released by the slow-conducting, unmyelinated C-polymodal nociceptors in a process known as an “axon reflex.” They are released into peripheral tissues from the peripheral terminals of the C-fibers, modulating the inflammatory process by “neurogenic inflammation.” [93–96] They are also released from the central terminals of the nociceptors into the dorsal horn of the spinal cord, where they modulate pain processing and spinal cord reflex activity. [97–99]
This neurophysiologic effect of spinal HVLAT manipulation seems to be force threshold-dependent. [31, 100] The threshold was found to lie somewhere between 450N and 500N for the thoracic spine and 400N for the lumbar spine. [101] When compared with data from biomechanical studies of spinal manipulation, [47] these forces would be sufficient to cause cavitation. The “SP” studies used “sham manipulation” as a control, consisting of a “low-velocity light-force thrust to the selected segment,” rather like a mobilization. This illustrates that zygapophyseal HVLAT manipulations that cause cavitation produce physiological effects, not demonstrable by electromyography, that are totally different from effects created by zygapophyseal manipulations that do not cause cavitation.
It has been proven that this neuropeptide release can occur only if cavitation is produced; however, it has also been proven to be a unique occurrence with manipulation of a zygapophyseal joint. These effects do not occur if HVLAT manipulation is applied to a peripheral joint such as the ankle joint. [102] This result indicates that this response to a spinal HVLAT manipulation, although obviously mediated by the nervous system, cannot be explained by simple mechanoreceptor-mediated reflex arcs. An equivalent force or pressure exerted directly to other parts of the musculoskeletal system such as the glutei [91, 102] or even directly to in vitro neutrophils in an attempt to manifest a cellular stress response [103] also fails to create a significant increase in plasma levels of SP. It would seem, therefore, that this effect of zygapophyseal HVLAT manipulation might depend on the unique anatomic location of these joints.
The zygapophyseal joints of the spine are in very close proximity to the DRG at each intervertebral segment. Badalemente et al [104] demonstrated that production of SP could be induced by mechanical stimulation of the DRG. From the aforementioned experiments conducted by Brennan et al, [31, 89–92, 102] it is likely that a form of stimulation of the DRG caused the production of SP. However, in view of biomechanical studies of HVLAT manipulation, [22, 25, 105–107] this stimulation could not realistically be mechanical.
Brennan et al did not provide a full explanation of the exact cause of the SP release but instead drew the reserved conclusion that “regardless of the mechanism whereby spinal manipulation primes phagocytic cells for an enhanced RB, it is a consistent response of cells from asymptomatic subjects receiving manipulation that is not observed from asymptomatic subjects receiving either sham manipulation or soft tissue mobilization. Therefore, it is likely that RB activity can be used as a physiological indicator that a true manipulative procedure of the thoracic spine (and the lumbar spine [92]) has been carried out. As clinical research involving the therapeutic effects of manipulation becomes more common, the need for a tool to verify that an appropriate sham procedure has been administered becomes crucial to understanding treatment efficacy. The monitoring of biological effects such as those described in this study may provide that tool.” [31] The efficacy of this temporarily enhanced RB as a “reliable quality control for monitoring the delivery of both a manipulation procedure and a control mobilization procedure in patients enrolled in a randomized control trial of spinal manipulative therapy” has since been shown. [91]
Disruption of articular or periarticular adhesions
The normal range of movement of any synovial joint has been termed the “physiological zone.” [108] Before the application of the “thrust” phase of an HVLAT manipulation, a “pre-load” force is applied. [47, 109] This involves taking the viscoelastic SF to a well-defined elastic recoil (which is particularly strong with small displacements), [49] characterized by increased stiffness. [53] Sandoz [108] described this as the “physiological barrier.” The additional impulse, which creates the (high-velocity) movement between the articular surfaces of the joint, is ultimately delivered to the SF. [54] It is known that when subjected to very high shear rates, liquids begin to behave mechanically like solids; for example, fracturing like a brittle solid. [110, 111] Cavitation occurs when the articular surfaces are separated through the elastic recoil of the SF above a critical velocity (vc), causing the SF to “fracture” or “crack” open like a solid. A proportion of the “cracking” noise during cavitation of SF may therefore be considered as synonymous with the “nucleation” or “inception” of the cavity [112, 113] (Figure 1).
It is interesting that the speed of force application during spinal HVLAT manipulation appears clinically relevant. It has been shown that less total force is required to produce cavitation when a fast rate of force application is used compared with a slow rate. [66] Thus, if the rate of force application is fast (v > > vc as in Fig 1), the total force applied to the manipulated joint will be less, and consequently, potentially safer (as long as the force is applied over a very short amplitude, ie, within the “physiological zone”).
A second “crack” cannot be produced until approximately 20 minutes after cavitation, because during this period gas remains in solution in the form of small bubbles that act as nuclei. [114, 115] This period is known as the “refractory period.” [3, 108] Any further tension simply reduces the pressure of the gas bubbles, leaving the liquid relatively undisturbed and under no influence of the tension. [3] Thus, during the refractory period, the SF offers little resistance to force, and a temporary increased range of motion is given to the joint. If a second attempt at “cracking” the joint is made during the refractory period, the joint will immediately be pushed toward the limit of anatomic integrity (the anatomic barrier of resistance), because the (fluid cohesion) elastic barrier of resistance has been temporarily eliminated by the initial crack. [116] Any further resistance to movement offered by this joint is from anatomic tissues.
Mierau et al [117] compared manipulation (“high velocity-low amplitude force, often accompanied by an audible cracking sound coming from the target joint”) and mobilization (“a gentle, often oscillatory, passive movement”) of the third metacarpophalangeal (MCP) joint. They found that the manipulated group demonstrated a significant temporary increase in passive MCP joint flexion over the mobilized group and concluded, “manipulation and mobilization are distinct therapies with different effects on joint function and should not be considered equivalent as they have been in the past.” It is likely that this “different effect” is solely due to the cavitation of the SF of the joint and not due to any mechanoreceptor-mediated muscular reflex response. This is because the collateral ligaments of the MCP joints play primary roles in joint stability, [118] and, hence, stability supplied by muscles would not be very significant. Any increase in range of motion would therefore be relatively independent of muscular influence.
Short-term increases in cervical range of motion immediately after HVLAT manipulation have previously been shown. [119–122] Lewit [123] examined the cervical spine of 10 patients before operation (most for abdominal surgery) and re-examined during anesthesia with myo-relaxants and intubation with artificial respiration. In all cases movement restriction remained unchanged and was even more easily recognizable under narcosis, because the patient was completely relaxed. He concluded, “the importance of this experiment is not only that it proves that movement restriction is (also) an articular phenomenon, but it also proves we are dealing with a mechanical obstacle in the joint.”
This “obstacle” may be due to meniscoids as mentioned previously, but this situation is more likely to present as a painful “acute locked” joint rather than just an asymptomatic “restriction.” The latter condition may simply be due to atmospheric pressure combined with the cohesive property of SF, creating strong elastic recoil. [2, 3, 49, 50, 54, 115] This finding suggests that the temporary increase in mobility of zygapophyseal joints after HVLAT manipulation found by Nilsson et al [122] may also be relatively independent of any neurophysiologic “reflex” effects on the motor system.
Manipulation of an intra-articular “adhesion” that is maintained only by atmospheric pressure and the cohesive behavior of the SF [54] has the potential only to temporarily increase range of motion. SF adhesion between articular surfaces (reducing mobility) cannot alone create a noxious stimulus. Burton et al [124] have shown that symptomatic improvement in patients with low-back pain treated with manipulation does not rely on alterations in mobility and is poorly correlated with increased sagittal mobility. Consequently, removal of this adhesion by breaking the “seal” created by the SF cannot explain pain-related or nonmechanical effects of zygapophyseal HVLAT manipulation.
It is also possible that after injuries to a zygapophyseal joint such as a capsular tear or a subchondral fracture, intra-articular hemorrhage could act as a precipitating factor for intra-articular fibrosis. [12] It is known from studies of animal and human knee joints that immobilization of the joint results in proliferation of intra-articular fat pads. [125, 126] Such adhesions could account for the stiffness of cervical joints evident on manual examination of inter-segmental motion as reported in the literature. [123, 127] These “adhesions,” however, are likely to be of a collagenous nature [125, 128] and would thus have viscoelastic properties. [40] If increased mobility of these tissues was required, they should be “stretched” at a slower rate than is involved in an HVLAT manipulation. [46, 129]
Unbuckling of motion segments that have undergone disproportionate displacements
This vague heading is assumed to be in relation either to realignment of joint “subluxations” or to previous speculations of the “replacement” of fragments from the nucleus pulposus of the intervertebral disks after HVLAT manipulation.
The concept that HVLAT manipulation “realigns” or “replaces” “misaligned” or “subluxed” joints is one of the oldest theories of spinal manipulation. [130] This theory was the likely reason that “bonesetters” gained their name. [20] One of the reasons for the conception of this theory is due to the audible joint “crack” caused by cavitation, which often conveniently coincides with immediate symptomatic relief. Before cavitation was widely accepted as the source of the crack, manipulating practitioners felt that they were “putting the bone back in place” (many patients still hold this concept after HVLAT manipulative treatment, and patient education to dispel these inaccurate beliefs is very necessary).
Recent biomechanical studies examining the motions of vertebrae after HVLAT manipulations show this “positional” theory to be false and merely demonstrate transient relative movements of the manipulated vertebrae associated with cavitation. [25, 105–107, 131] Radiographs, computed tomography, and magnetic resonance imaging scans have been shown to be an unreliable method for diagnosing back pain. [132–136] Therefore, with regard to sources of spinal pain that respond to HVLAT manipulation, “subluxations” or “misaligned vertebrae” appear to be an epiphenomenon.
The theory of the “replacement” of fragments of the nucleus pulposus of the intervertebral disks after HVLAT manipulations, as advocated by Cyriax [137] and those who follow his approach, [138] is another unlikely theory to explain both the cracking sound and symptom-relieving effects of spinal HVLAT manipulations. If this were the case, it would not be possible to produce a joint “crack” from many other synovial joints in the body including MCP, sacroiliac, occipito-atlantal, and atlanto-axial joints, where there are no intervertebral disks (or any similar structures) present. From this theory, it also should not be possible to produce a “crack” from all spinal segments, because a displaced fragment of nucleus pulposus (that needs “replacing”) would not occur in all spinal segments of every person.
An acute cervical or lumbar disk herniation has generally been regarded as a contraindication to HVLAT manipulation of the herniated segment, [139, 140] especially in the presence of severe or progressive neurologic deficit. However, a long-term beneficial effect of manipulation on symptomatic lumber disk herniation has been demonstrated, showing improvements in leg pain, back pain, and self-reported disability. [141] It is unlikely that these improvements were due to structural changes to the disk because, although there have been case reports of apparent reductions of small lumbar disk protrusions after manipulation, [142] computed tomography imaging and myelography have failed to demonstrate persistent reduction of disk protrusion after manipulation. [143–145]
The nucleus pulposus is rich in hydrophilic (water-binding) glycosaminoglycans (in the young adult). During loading of the spine, it acts hydrostatically, [146] allowing a uniform distribution of pressure throughout the disk. Therefore the disk acts as a cushion, storing energy and distributing loads. [147] The nucleus pulposus also has viscoelastic (rate-dependent) properties, causing greater stiffness (resistance to deformity) of the tissue at higher strain rates. Recent biomechanical evidence [148] suggests that variable transient changes in intradiscal pressure do arise during spinal manipulation, but the clinical significance of this is unknown. Excessive displacements of the fluid nucleus, from the area of greatest to the area of least load, may also result from the adoption of prolonged static positions. [148] Thus it would be logical that if a nuclear fragment was to be gradually “encouraged” to safely return toward the center of the intervertebral disk, then a more static or very low velocity maneuver should be sought. Bogduk and Jull [11] reviewed the possibility of the replacement of an “intra-discal nuclear displacement” by manipulative therapy. They described a series of combined movements of the “affected motion segment,” emphasizing the word “progressively,” thereby discounting a high-velocity procedure.
The existence of gas bubbles in synovial joints (after cavitation) has been demonstrated by radiography as a dark, intra-articular radiolucent region since early in the twentieth century. [3, 114, 149–152] Magnetic resonance imaging scans have shown that lumbar HVLAT manipulation gaps zygapophyseal joints and increases dimensions of the intervertebral foramen. [16, 153] These studies provide clear evidence that the anatomic source of the cracking sound associated with spinal HVLAT manipulations is the zygapophyseal joints and not the intervertebral disks, and that the audible “crack” is associated with cavitation of the SF.
Summary of previous theories
From this information, there seem to be unique neurophysiological effects associated with cavitation from a zygapophyseal HVLAT manipulation in subjects with and without symptoms. [31, 82–92, 102]
Some reasonable theoretical “mechanical” explanations have been offered for beneficial mechanisms of zygapophyseal HVLAT manipulation for acute low back pain. [11] However, because of the non-nociceptive behavior of chronic low back pain (involving “central” pain mechanisms), clinical improvements in this condition from manipulation cannot be explained by “mechanical” theories alone or by any published hypothesis. [7, 25] There are also non–symptom-related neurophysiologic effects that also cannot be sufficiently explained by any published theory. [31, 82–87, 89–92, 102]
Conclusion
There seem to be 2 totally separate modes of action from zygapophyseal HVLAT manipulation. The intra-articular “mechanical” effects of zygapophyseal HVLAT manipulation seem to be absolutely separate from, and irrelevant to, the occurrence of observed “neurophysiologic” effects. Cavitation should not be an absolute requirement for the mechanical effects to occur but may be a reliable indicator for successful joint gapping.
When clinical efficacy has been previously assessed, spinal mobilization and HVLAT manipulation have been grouped together as equivalent interventions. [5, 6] This has always implied that these 2 interventions have identical biologic effects. There may now be enough theoretical reasons to assess mobilization and manipulation as separate clinical entities.
Acknowledgments
Thanks for useful comments are due to Prof. John Blake, University of Birmingham (cavitation), and Mick Thacker, Kings College, London (neurophysiology). Thanks are also due to Will Podmore, British School of Osteopathy, London, for proofreading the manuscript.

Return to CHIROPRACTIC SUBLUXATION
Since 6-02-2002


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |